Anatomically Contoured Head for THA
The use of ceramic bearings in hip arthroplasty has been steadily rising in the last few years. Ceramic is now the preferred femoral head material in hip replacement. There is a consensus that the biological advantages of ceramic contributes to improve clinical outcomes.
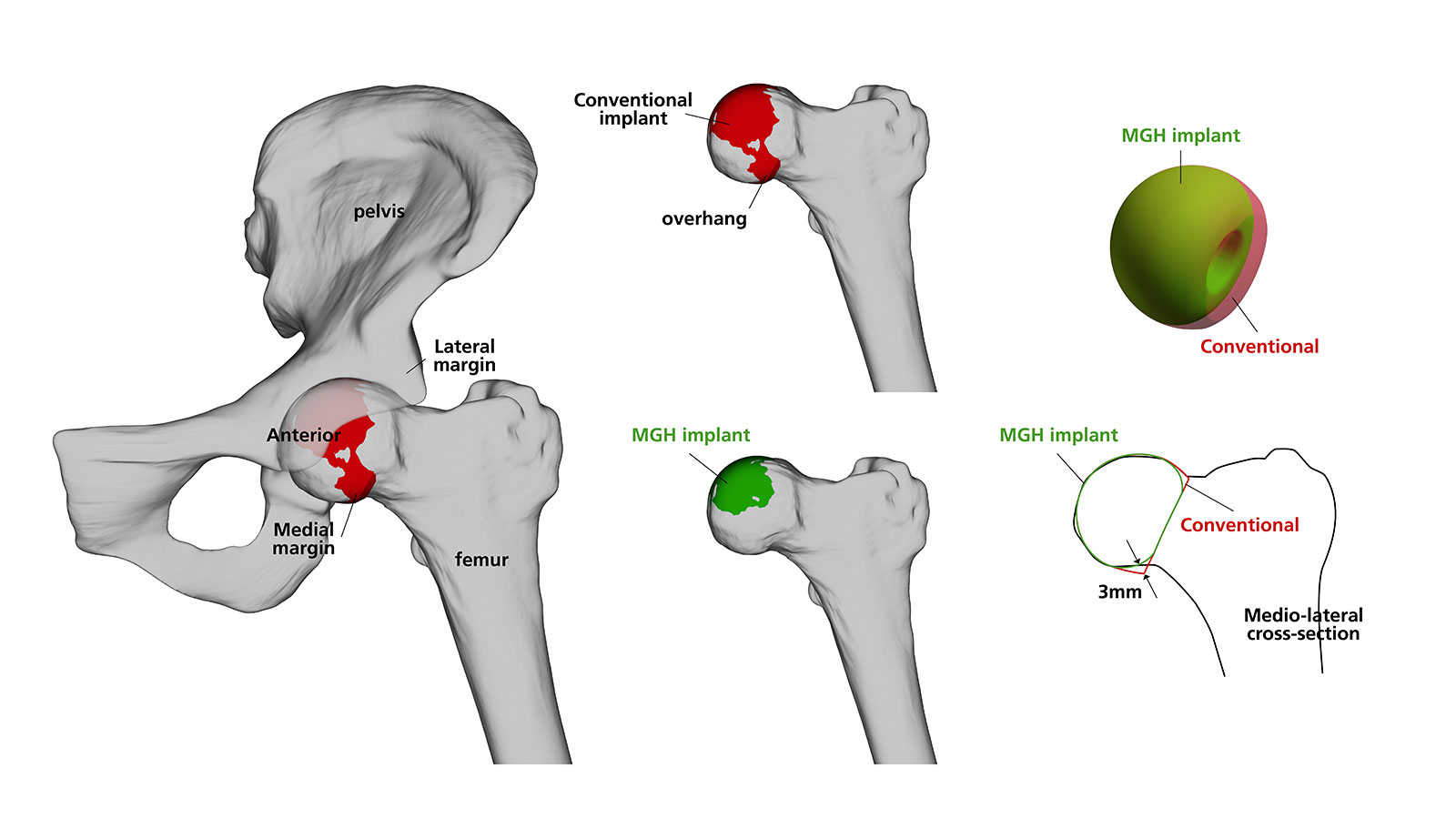
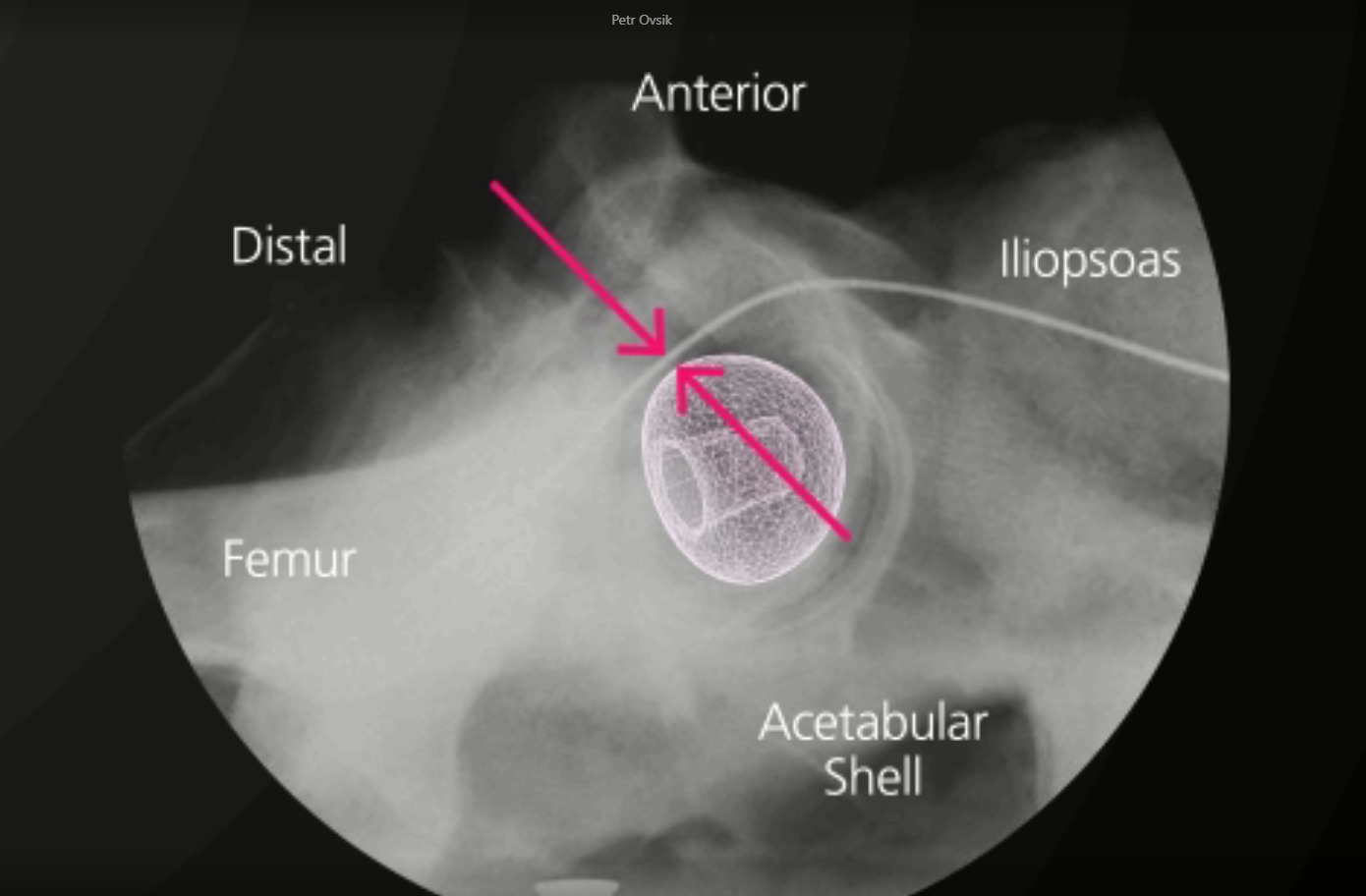
BIOLOX CONTOURA® is a contoured ceramic femoral head mimicking the anatomical shape of the proximal femur.
Designed with the goal of:
Reducing the volume of material exposed to soft tissue
Reducing the potential for interaction with the surrounding tissues
Development
The BIOLOX CONTOURA® head was conceived and developed at the Harris Orthopaedics Laboratory at Massachusetts Hospital in Boston, USA.
Research on the implant began in 2012, led by Drs. Ohrun Muratoglu and Kartik Mangudi Varadarajan, PhD, Associate Director for the TIRC at MGH, and Assistant Professor of Orthopedic Surgery at Harvard, together with Drs. Harry Rubash, Henrik Malchau, and Andrew Freiberg. The research team worked with the business development unit of Partners HealthCare Innovation, and partnered with CeramTec to design and test the anatomically contoured ceramic head.
The biocompatibility of alumina matrix composites like BIOLOX®delta has been proven, clinically, in in-vitro studies and in in-vivo animal studies. The BIOLOX®delta particles fail to stimulate an inflammatory response and do not cause any DNA damage or oxidative stress in human cells in clinically-relevant doses. In other words, the material and its debris are not cytotoxic and/or genotoxic.
Excellent biocompatibility
Low immunological response
High wear resistance
Reduced bacterial adhesion
Safe in terms of metal ion release
Best clinical outcomes
High potential for cost-effectiveness in THA
Recent in-vitro studies and analyses of explants have demonstrated that the bacterial adhesion is reduced on BIOLOX® ceramic surfaces. Additionally, studies showed a reduced biofilm formation on BIOLOX® surfaces compared to metal and polymer surfaces.
The biocompatibility of alumina matrix composites like BIOLOX®delta has been proven, clinically, in in-vitro studies and in in-vivo animal studies. The BIOLOX®delta particles fail to stimulate an inflammatory response and do not cause any DNA damage or oxidative stress in human cells in clinically-relevant doses. In other words, the material and its debris are not cytotoxic and/or genotoxic.
Excellent biocompatibility
Low immunological response
High wear resistance
Reduced bacterial adhesion
Safe in terms of metal ion release
Best clinical outcomes
High potential for cost-effectiveness in THA
Recent in-vitro studies and analyses of explants have demonstrated that the bacterial adhesion is reduced on BIOLOX® ceramic surfaces. Additionally, studies showed a reduced biofilm formation on BIOLOX® surfaces compared to metal and polymer surfaces.
Large-diameter
With the BIOLOX CONTOURA®, the large-diameter profile of a conventional implant is retained in the hemispherical portion above the equator. However, the distal portion of the femoral head below the equator is contoured using a smaller radius to reduce the volume of material exposed to the soft tissue.
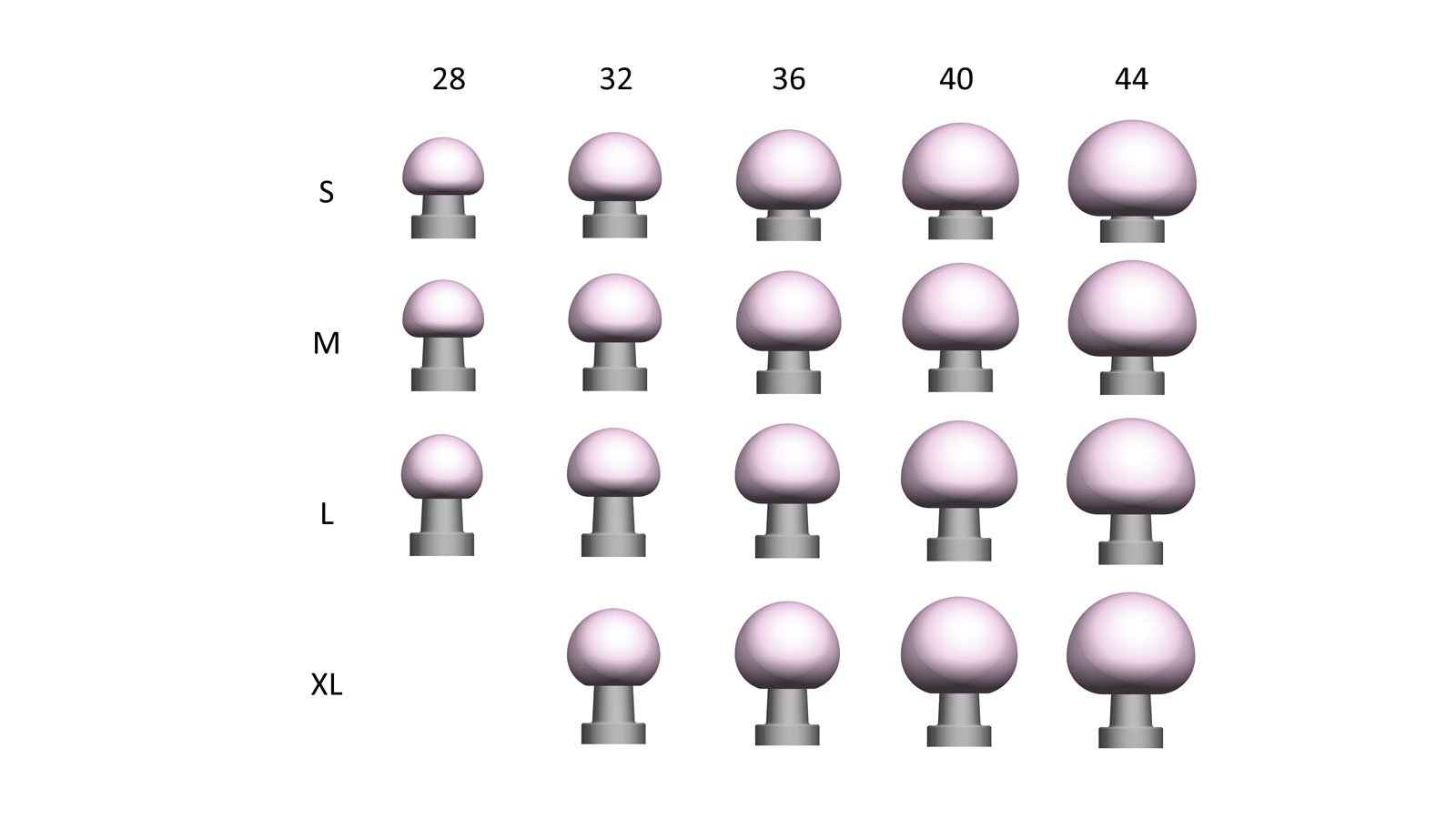
Available Sizes
BIOLOX CONTOURA® heads are available in diameters 28, 32, 36, 40 and 44mm and for the neck lengths S, M, L, XL.
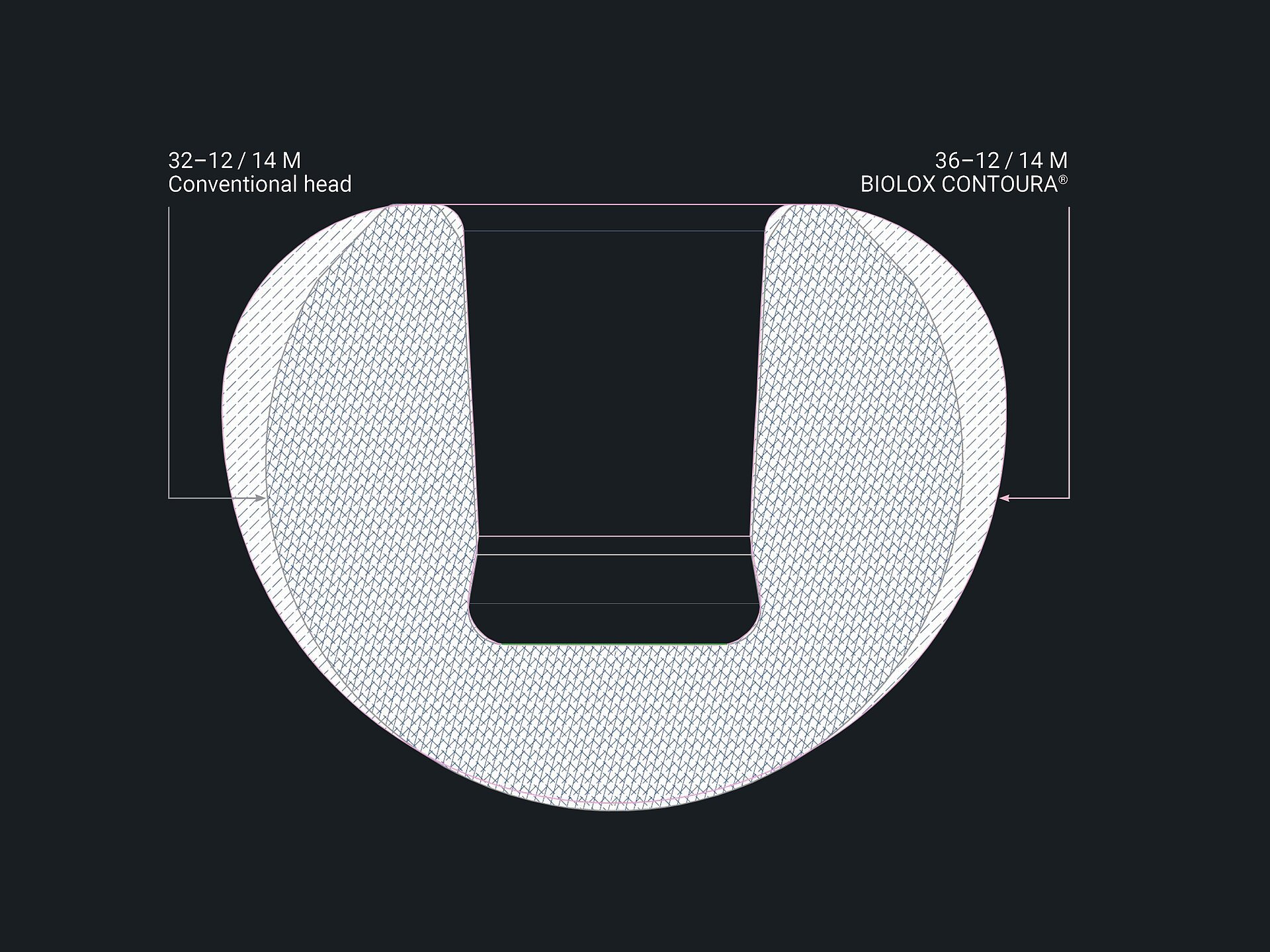
An overlay of a 36mm BIOLOX CONTOURA® head and a 32mm conventional ceramic femoral head shows the volume reduction of BIOLOX CONTOURA®. See volume reduction here.