Implant allergy testing has emerged as a crucial tool in addressing the increasing number of patients presenting with adverse reactions to implanted medical devices. As the use of implants in medical practice continues to grow, so too does the need for precise diagnostic methods to identify and address potential allergic reactions and to distinguish them from other inflammatory reactions. These reactions can range from localized inflammation to systemic immune responses, underscoring the importance of accurate and timely testing to mitigate complications and ensure patient safety.
The causes of implant allergies are multifaceted. Common triggers include hypersensitivity to metals such as nickel, cobalt, and chromium, which are often used in orthopedic and dental implants. Non-metal components, such as polymers, ceramics, and adhesives, can also provoke allergic responses in susceptible individuals. These reactions are typically mediated by antigen-specific T cells in the form of the so-called delayed-type hypersensitivity. Factors such as a patient’s genetic predisposition, history of allergies or atopic diseases, and the specific materials used in the implant play significant roles in the development of these reactions.
Symptoms of implant allergies vary widely and can affect both the implant site and the patient’s overall health. Localized symptoms may include redness, swelling, warmth, pain, and delayed wound healing, often mimicking signs of infection. Systemic symptoms, though less common, can include fatigue, joint pain and skin rashes. These symptoms can significantly impact a patient’s quality of life, making timely and accurate diagnosis crucial.
The Munich Implant Allergy Research Group in the Clinic of Dermatology and Allergology at the Ludwig-Maximilians-University has been running a special implant allergy consultation for many years and has seen over 4000 patients with a suspected implant allergy during this period. With this clinical and diagnostic experience, we are able to explore, to develop, and to refine testing methodologies to ensure patient safety and optimal outcomes. Our mission is to bridge the gap between clinical practice and cutting-edge research, enabling healthcare providers to make informed decisions based on robust diagnostic tools. Our multidisciplinary approach combines dermatology, immunology, orthopedics, and laboratory medicine, creating a comprehensive framework for diagnosing and managing implant allergies.
This article delves into four primary testing modalities:
patch testing,
lymphocyte transformation assay (LTT),
IL-1α/IL-1β stimulation test, and
genetic testing.
Each method offers unique insights and can play a special role in a cohesive diagnostic strategy. By sharing our perspectives and experiences, we aim to shed light on the potential and challenges of these techniques, contributing to the broader understanding and advancement of implant allergy testing.
Patch testing is often the first diagnostic step when an implant allergy is suspected. This method involves applying small quantities of suspected allergens, such as metals or chemicals commonly used in implants, to the patient’s skin under occlusive patches. After 48-72 hours, the skin is examined for signs of an allergic reaction (Figure 1).
Importantly, a late reading, performed after 96 hours or later, can significantly enhance the test's diagnostic accuracy. Delayed-type hypersensitivity reactions often require more time to manifest, and a late reading ensures that subtle or slow-developing responses are not missed.
This practice reduces the risk of false-negative results and provides a more comprehensive assessment of the patient's reactivity.
From our perspective, patch testing is particularly valuable for identifying delayed hypersensitivity reactions, especially to metals like nickel, cobalt, and chromium. However, its limitations cannot be overlooked. The results are not always predictive of systemic reactions, particularly those that occur deep within tissues around the implant. Furthermore, factors such as individual skin reactivity and the potential for irritant reactions can complicate interpretation.
To mitigate these limitations, we’ve adopted a meticulous approach to patient history-taking and use a standardized panel of allergens. When possible, we also consider environmental and occupational exposures that might influence the results. For example, we employ advanced testing panels tailored to specific implant materials, which include not only metals but also polymers, ceramics, and composite materials.
Despite its constraints, patch testing remains a cornerstone for initial screening due to its accessibility and established methodology.
For our patients in the special implant allergy consultation, we supplement the epicutaneous test with the lymphocyte transformation assay (LTT). This blood-based test evaluates whether a patient’s lymphocytes react to specific allergens by measuring cell proliferation in response to the suspected substances.
Our experience with LTT has highlighted its superior sensitivity for detecting systemic immune responses compared to patch testing. For example, we have identified a quite high number of patients with positive LTT tests to nickel, cobalt or chromium when patch tests to these substances were negative.
The test itself involves isolating lymphocytes from the patient’s blood and exposing them to potential allergens in a controlled laboratory environment. Proliferation rates are then quantified using advanced markers, such as incorporation of fluorescent dyes. These techniques allow us to measure even subtle immune responses, providing invaluable data for complex cases.
However, LTT is not without challenges. It requires specialized laboratory equipment and expertise, and its cost can be a barrier for some patients. The interpretation of results also demands caution, as not all lymphocyte proliferation correlates directly with clinical symptoms. To overcome these hurdles, we are evaluating our LTT tests in larger studies and also on large groups of healthy control blood donors and perform continuous training to ensure that LTT is applied judiciously and interpreted accurately (Figure 2).
The IL-1α/IL-1β stimulation test is an emerging method for assessing implant allergies by measuring the inflammatory response to specific materials. This test evaluates the release of interleukin-1α and interleukin-1β, two key cytokines involved in inflammation and immune regulation, when patient cells are exposed to implant materials.
This method provides valuable insights into the pro-inflammatory potential of implants, especially in cases where conventional tests yield inconclusive results. The IL-1α/IL-1β stimulation test can in special cases be useful for assessing materials considered biocompatible, such as titanium or certain polymers, as it can detect low-grade inflammation that may contribute to implant failure or chronic symptoms.
However, the test is not without its limitations. While it offers a more direct measurement of inflammatory potential, it is still a laboratory-based test requiring sophisticated equipment and technical expertise. Moreover, the clinical relevance of elevated IL-1α/IL-1β levels is not fully understood, as inflammation can result from non-specific stimuli or unrelated conditions.
Interpretation of results must therefore be done cautiously and in conjunction with other diagnostic findings. To address these challenges, we advocate for standardized protocols and further research into the clinical significance of IL-1α/IL-1β measurements in larger and especially prospective studies.
The advent of genetic testing has opened new possibilities for predicting implant adverse reactions, particularly in the pre-implantation phase. Variants in genes related to immune function, such as HLA (human leukocyte antigen) alleles, have been associated with an increased risk of hypersensitivity reactions to certain materials.
Among the genetic tests, the detection of IL1RN polymorphisms stands out as a significant advancement in implant allergy diagnostics. IL1RN encodes the interleukin-1 receptor antagonist, a critical regulator of inflammatory responses. Specific polymorphisms in this gene can influence the balance between pro-inflammatory and anti-inflammatory signals, predisposing individuals to exaggerated immune responses when exposed to implant materials (Figure 3). By identifying these genetic variants, we can better predict a patient’s likelihood of developing implant-related inflammation or allergy. This predictive capacity represents a crucial advantage, allowing for preemptive measures, such as selecting alternative materials or enhancing postoperative monitoring.
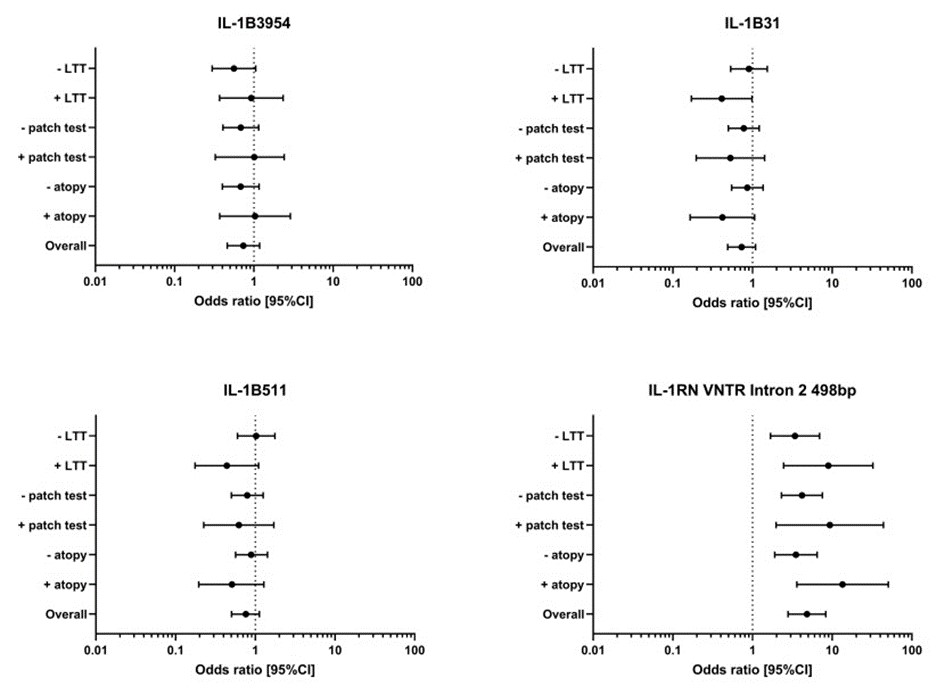
Figure 3:Odds ration (and 95% confidence interval CI) of the IL-1B polymorphism IL-1B 3954, IL-1B 31, IL-1B 511 and the IL-!RN VNTR Intron 2 498bp allele are given for the risk analysis of the symptomatic arthroplasty patient group against the control group overall and also for the subgroups atopy (yes/no), patch test to metal (positive/negative) and LTT to metal (positive/negative) (from Summer et al 2022)
Our approach to genetic testing involves analyzing patient DNA for specific polymorphisms associated with immune response. For example, variations in cytokine-producing genes may predict inflammatory responses to polymers or ceramics. By integrating these findings with clinical data, we aim to create a comprehensive risk assessment model.
However, this approach also raises ethical and practical questions. How should genetic information be communicated to patients? What safeguards are needed to prevent misuse of genetic data? These are questions we grapple with as we navigate the integration of genetic testing into routine clinical practice.
While each of these testing methods has its strengths, their true potential lies in their combined application. For instance, a patient with suspected implant allergy might undergo patch testing to identify surface allergens, followed by LTT for systemic validation, and genetic testing for predictive insights. By integrating these modalities, we can achieve a more accurate and holistic understanding of the patient’s immune response.
Additionally, we are exploring the use of emerging technologies, such as proteomic and metabolomic analyses, to complement traditional testing. These advanced techniques will perhaps allow us in the future to identify biomarkers that will provide further insight into immune activation and inflammation.
As a group deeply invested in the field of implant allergy, we strive to combine the strengths of the three modalities—patch testing, LTT, and genetic testing—into a cohesive diagnostic strategy. Our vision is to create a patient-centric approach that emphasizes early detection, accurate diagnosis, and tailored interventions.
We advocate for multidisciplinary collaboration, involving dermatologists, immunologists, orthopedic surgeons, and laboratory specialists. Through such collaboration, we can provide comprehensive care and advance our understanding of implant allergies.
Looking forward, we see a future where genetic testing becomes a routine part of pre-implantation assessment, and innovative biomarkers further enhance the sensitivity and specificity of tests like LTT. Meanwhile, patch testing will likely retain its role as a practical, first-line diagnostic tool.
Conclusion
Implant allergy testing is a dynamic and evolving field. Our Munich Implant Allergy Research Group remains committed to refining and advancing testing methodologies to ensure that patients receive the best possible care. By combining traditional approaches like patch testing with cutting-edge techniques like LTT, and genetic testing, we aim to set a gold standard in implant allergy diagnostics and management.
References
1. Bracey DN, Hegde V, Johnson R, Kleeman-Forsthuber L, Jennings J, Dennis D. Poor Correlation Among Metal Hypersensitivity Testing Modalities and Inferior Patient-Reported Outcomes After Primary and Revision Total Knee Arthroplasties. Arthroplasty Today. 2022 Oct 31;18:138-142.
2. Carossino AM, Carulli C, Ciuffi S, Carossino R, Zappoli Thyrion GD, Zonefrati R, Innocenti M, Brandi ML.Hypersensitivity reactions to metal implants: laboratory options. BMC Musculoskelet Disord. 2016 Nov 23;17(1):486.
3. Costa MD, Donner S, Bertrand J, Pop OL, Lohmann CH. Hypersensitivity and lymphocyte activation after total hip arthroplasty. Orthopadie (Heidelb). 2023 Mar;52(3):214-221.
4. de Graaf NPJ, Bontkes HJ, Roffel S, Kleverlaan CJ, Rustemeyer T, Gibbs S, Feilzer AJ. Non-heat inactivated autologous serum increases accuracy of in vitro CFSE lymphocyte proliferation test (LPT) for nickel. Clin Exp Allergy. 2020 Jun;50(6):722-732.
5. Fischer, M., & Maier, S. (2020). Advances in genetic testing for implant allergies: A clinical perspective. Journal of Biomedical Materials Research Part A, 108(4), 945-956.
6. Goodman, S. B., et al. (2019). Cytokine profiles and their diagnostic potential in implant-related complications. Clinical Orthopedics and Related Research, 477(5), 1021-1034.
7. Hallab, N. J., & Jacobs, J. J. (2009). Biologic effects of implant debris. Bulletin of the NYU Hospital for Joint Diseases, 67(2), 182-188.
8. Kimber I, Basketter DA. Allergic Sensitization to nickel and Implanted Metal Devices: A Perspective. Dermatitis. 2022 Nov-Dec 01;33(6):396-404.
9. Lützner J, Beyer F, Lützner C, Thomas P, Summer B. Increased inflammatory response is associated with less favorable functional results 5 years after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2023 Apr;31(4):1316-1322.
10. Oppel E, Kapp F, Böhm AS, Pohl R, Thomas P, Summer B. Contact sensitization to iron: A potentially underestimated metal allergen and elicitor of complications in patients with metal implants. Contact Dermatitis 2022;86(6):531-538
11. Peacock CJH, Fu H, Asopa V, Clement ND, Kader D, Sochart DH. The effect of nickel hypersensitivity on the outcome of total knee arthroplasty and the value of skin patch testing: a systematic review. Arthroplasty. 2022 Sep 2;4(1):40
12. Ständer S, Oppel E, Thomas P, Summer B. Evaluation of lymphocyte transformation tests as compared with patch tests in nickel allergy diagnosis. Contact Dermatitis 2017;76(4):228-34
13. Richards LJ, Streifel A, Rodrigues JM. Utility of Patch Testing and Lymphocyte Transformation Testing in the Evaluation of Metal Allergy in Patients with Orthopedic Implants. Cureus. 2019 Sep 25;11(9):e5761.
14. Schalock PC, Crawford G, Nedorost S, Scheinman PL, Atwater AR, Mowad C, Brod B, Ehrlich A, Watsky KL, Sasseville D, Silvestri D, Worobec SM, Elliott JF, Honari G, Powell DL, Taylor J, DeKoven J. Patch Testing for Evaluation of Hypersensitivity to Implanted Metal Devices: A Perspective From the American Contact Dermatitis Society. Dermatitis. 2016 Sep-Oct;27(5):241-7.
15. Schalock, P. C., & Thyssen, J. P. (2018). Metal hypersensitivity reactions to implants: A review and proposed screening algorithm. Contact Dermatitis, 79(1), 1-14.
16. Summer B, Lill D, Remmel K, Schraml A, Schopf C, Banke IJ, Kuechenhoff H, Maierhofer T, Endres S, Thomas P. An interleukin-1 polymorphism additionally intensified by atopy as prognostic factor for aseptic non-mechanical complications in metal knee and hip arthroplasty. Frontiers Immunology 2022; 13:1050315
17. Thomas B, Benedikt M, Alamri A, Kapp F, Bader R, Summer B, Thomas P, Oppel E. The role of antibiotic-loaded bone cement in complicated knee arthroplasty: relevance of gentamicin allergy and benefit from revision surgery - a case control follow-up study and algorithmic approach. J Orthop Surg Res 2020;15(1):319
18. Thomas P, Arenberger P, Bader R, Bircher AJ, Bruze M, de Graaf N, Hartmann D, Johansen JD, Jowitz-Heinke A, Krenn V, Kurek M, Odgaard A, Rustemeyer T, Summer B, Thyssen P. A literature review and expert consensus statement on diagnostics in suspected metal implant allergy. J Eur Acad Dermatol Venereol 2024 Aug;38(8):1471-1477.




