Inflammation is part of the innate biological response to infectious or non-infectious agents. It is a non-specific, essential defense mechanism against injury or intrusion by pathogenic micro-organisms, endogenous (e.g., gout crystals) or exogenous (e.g., implant wear debris) non-biological products.
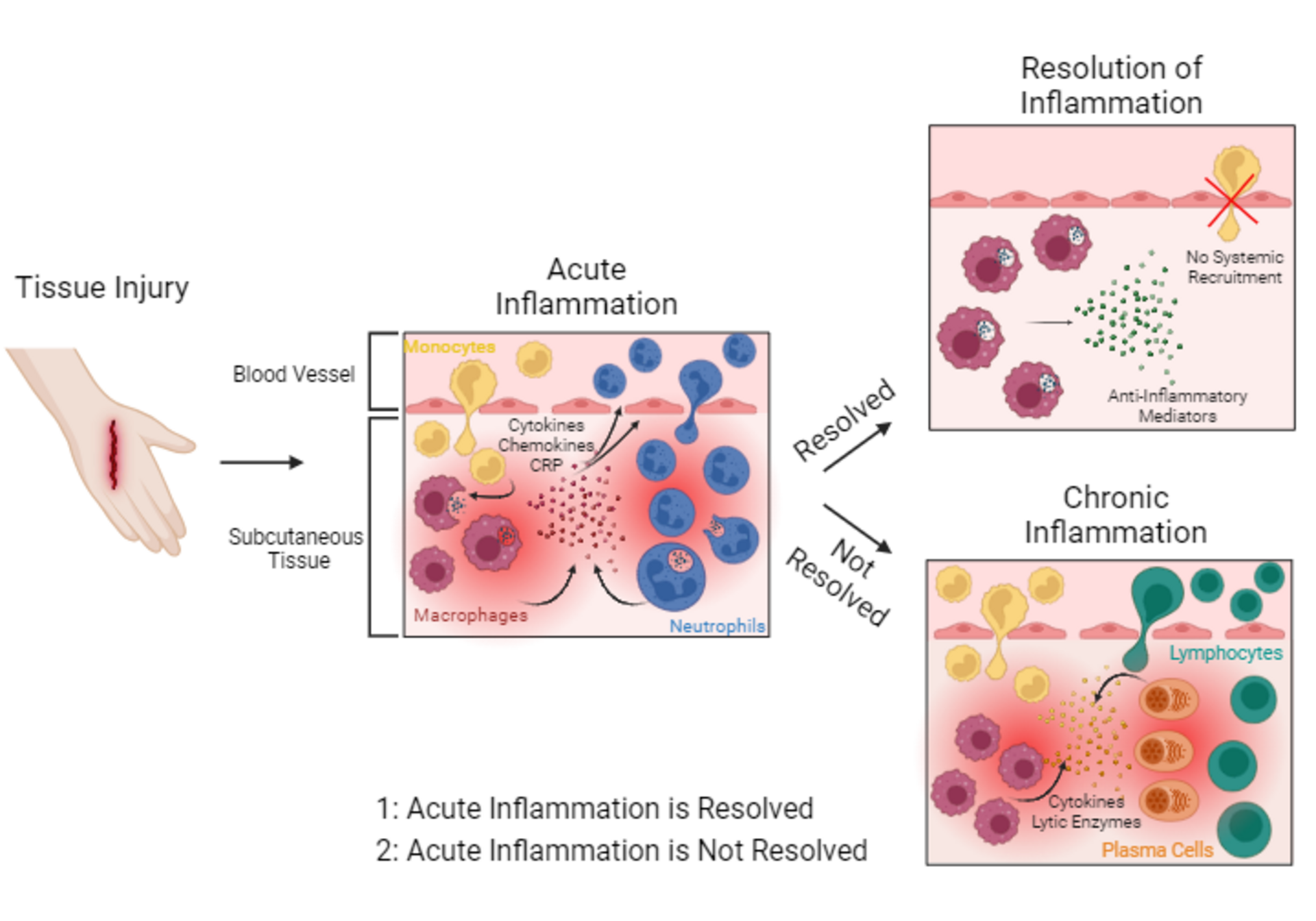
Skin injury results in the disruption of subcutaneous tissue and blood vessels. Tissue-resident macrophages (dark red cells) recognize damaged tissue particles and become activated. Activated macrophages start to engulf and digest damaged tissue particles. Furthermore, they release pro-inflammatory mediators such as cytokines, chemokines, and acute phase proteins such as C-reactive protein (CRP) (red dots) into the injured tissue. These pro-inflammatory mediators attract other immune cells to the site of tissue injury. Monocytes (yellow cells) and neutrophils (blue cells) migrate into injured tissue. Monocytes differentiate into macrophages. Neutrophils start to engulf and digest damaged tissue particles. Once damaged tissue particles are removed, tissue healing, and resolution of inflammation are induced. Resolution of inflammation: For the resolution of inflammation, macrophages release anti-inflammatory cytokines (green dots), stopping the pro-inflammatory response and the systemic recruitment of immune cells into the tissue. However, if the acute inflammatory response does not resolve it may progress from sub-acute to chronic inflammation.
Chronic inflammation is characterized by permanent migration of immune cells such as monocytes, lymphocytes (green cells) and plasma cells (orange cells) into the tissue, constantly releasing lytic enzymes and cytokines (yellow and orange dots), thus resulting in a nonresolving, persistent, chronic tissue inflammation.
Skin injury results in the disruption of subcutaneous tissue and blood vessels. Tissue-resident macrophages (dark red cells) recognize damaged tissue particles and become activated. Activated macrophages start to engulf and digest damaged tissue particles. Furthermore, they release pro-inflammatory mediators such as cytokines, chemokines, and acute phase proteins such as C-reactive protein (CRP) (red dots) into the injured tissue. These pro-inflammatory mediators attract other immune cells to the site of tissue injury. Monocytes (yellow cells) and neutrophils (blue cells) migrate into injured tissue. Monocytes differentiate into macrophages. Neutrophils start to engulf and digest damaged tissue particles. Once damaged tissue particles are removed, tissue healing, and resolution of inflammation are induced. Resolution of inflammation: For the resolution of inflammation, macrophages release anti-inflammatory cytokines (green dots), stopping the pro-inflammatory response and the systemic recruitment of immune cells into the tissue. However, if the acute inflammatory response does not resolve it may progress from sub-acute to chronic inflammation.
Chronic inflammation is characterized by permanent migration of immune cells such as monocytes, lymphocytes (green cells) and plasma cells (orange cells) into the tissue, constantly releasing lytic enzymes and cytokines (yellow and orange dots), thus resulting in a nonresolving, persistent, chronic tissue inflammation.
Based on the onset and duration of the symptoms, inflammation can be categorized as acute, sub-acute, or chronic. Acute inflammation starts immediately after a specific injury or infection and typically lasts only a few days. It is characterized by the release of soluble immune mediators including acute phase proteins such as C-reactive protein, cytokines, and chemokines attracting neutrophils and macrophages to the area of injury. These cells initiate the healing process or the elimination of the infectious or non-infectious intruder. The resolution of the inflammatory process involves the controlled production of mediators, and the decrease of chemokine concentrations to reduce and stop the recruitment of white blood cells.1 If the acute inflammation does not resolve, it may evolve from sub-acute (two to six weeks) to chronic inflammation, which may last for months or even years. Chronic inflammation is sustained by the continued recruitment and infiltration of mononuclear leucocytes such as macrophages, lymphocytes, and plasma cells releasing cytokines and lytic enzymes which may damage the tissue again, thus prolonging the tissue injury followed by secondary repair often associated with fibrosis and granulomatous reactions.2
Causes of dysregulation and prolongation of the inflammatory process include failure to eliminate the causative agent, which is either a resistant microbial pathogen or a substance that cannot be phagocytosed or broken down enzymatically (such as wear debris from articulating surfaces) as well as factors causing oxidative stress (increased release of free radicals, advanced glycation end products (AGEs), urate crystals, oxidized lipoproteins, etc.).
In the context of orthopedic implants, the pathogenesis of chronic inflammation often involves a complex cascade of immune reactions related to the implant material, to the surgery, to an associated (low-grade) infection, and/or to the patient’s underlying condition. Discerning an aseptic chronic inflammatory syndrome from a chronic low-grade infection is difficult, as they are often concomitant and related. The presence of the implant itself as a foreign body constitutes a major risk factor for the onset, prolongation, and persistence of both inflammation and infection. As described above, the protracted inflammatory process may cause tissue damage, fibrosis, and granulomatosis. The natural immunological defense may fail to eliminate microorganisms in this compromised environment. Additionally, bacteria are attracted to implant surfaces to which they may attach, and subsequently colonize and form biofilms, acting as a physical barrier protecting the bacteria from immunocytes and antibiotics.3 These biofilms may also prohibit osseointegration and eventually lead to implant loosening. Again, the differentiation
between aseptic and low-grade septic loosening is often difficult and interrelated.
Clinical symptoms and diagnostic tests for chronic Inflammation
Symptoms of chronic inflammation may vary from local pain, swelling, and dysfunction to more generalized arthralgia, myalgia, and malaise. Systemic symptoms may include subfebrile fever, fatigue, weight loss or gain, neurological and gastrointestinal symptoms, higher susceptibility to infection, insomnia, anxiety, and depression. Chronic inflammation represents a threat to the global health of the individual and is associated with higher morbidity and mortality.
Currently there are no specific laboratory tests for the diagnosis of chronic inflammation. Good serum markers of inflammation include hs (high-sensitivity) C-reactive protein and fibrinogen but are not specific to chronic inflammation; they are also elevated in cases of acute inflammation or infection. These standard tests are inexpensive and can be performed in routine medical laboratories. Specific tests of proinflammatory cytokines such as interleukin 6 (IL-6) are more expensive, not routinely available, and sometimes difficult to interpret.
Imaging techniques may play an important role in the diagnosis and monitoring of chronic inflammation.4 Besides the conventional and widely used X-ray, CT, MRI, PET/CT, and the more specialized FDG-PET-CT scintigraphy with Tc, Ga, or In-white blood cells, new, highly sophisticated imaging techniques are being developed, to localize sites of inflammation in detail and monitor activity during treatment. These techniques include molecular and multimodal imaging, optical imaging of immune cell trafficking, photoacoustic imaging, MRI sensors for biomarkers, and hyperpolarized MRI for the detection of oxidative stress.5 These new techniques are expected to facilitate the differential diagnosis between chronic inflammation and low-grade infection in the future.
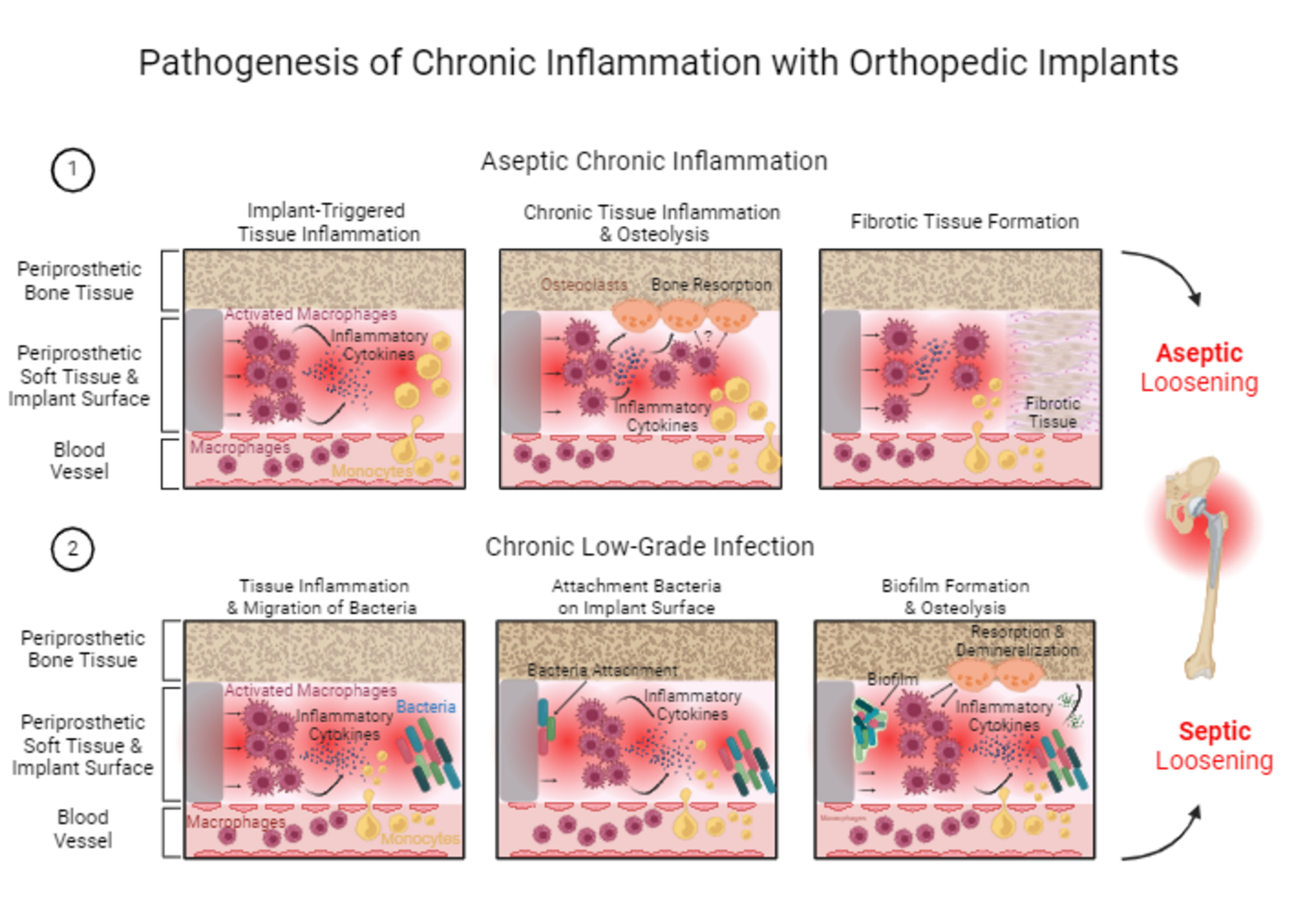
During aseptic chronic inflammation tissue-resident macrophages (dark red spiky-shaped cells) in periprosthetic tissue are permanently activated by the implant material surface. The activated macrophages release pro-inflammatory cytokines (blue dots) and trigger chronic tissue inflammation. Monocytes (yellow cells) are permanently attracted and migrate into periprosthetic tissue. Monocytes differentiate into macrophages, which are then getting activated by material surface promoting the chronic tissue inflammation. During chronic tissue inflammation, the released pro-inflammatory cytokines and activated macrophages themselves activate osteoclasts (orange cells), which then cause bone resorption and periprosthetic osteolysis. Furthermore, chronic inflammation results in the generation of fibrotic tissue (pink tissue, with pink dots), thus replacing functional periprosthetic soft tissue with fibrotic tissue. Aseptic chronic inflammation with implant-triggered tissue inflammation, osteolysis and fibrotic tissue formation is associated with the development of aseptic implant loosening.
During chronic low-grade infection, macrophages in periprosthetic tissue (dark red spiky-shaped cells) are constantly activated by the implant material surface and release pro-inflammatory cytokines (blue dots). Monocytes (yellow cells) permanently migrate into periprosthetic tissue and differentiate into macrophages. The chronic and compromised tissue inflammation favors the migration of bacteria into periprosthetic tissue, additionally fueling tissue inflammation. The compromised inflammatory response in the tissue is not able to clear bacteria, which then allows them to attach to the implant surface. Once attached to the implant surface, bacteria form a biofilm, which makes them resistant to immune cells and to antibiotic treatment. Activated macrophages and pro-inflammatory cytokines furthermore activate osteoclasts, which then start to resorb bone, resulting in periprosthetic osteolysis. Additionally, bacteria from biofilm release acidic factors (green dots), which cause bone demineralization. Chronic low-grade infection with chronic tissue inflammation, bacteria migration, biofilm formation, osteolysis and bone demineralization is associated with the development of septic implant loosening.
During aseptic chronic inflammation tissue-resident macrophages (dark red spiky-shaped cells) in periprosthetic tissue are permanently activated by the implant material surface. The activated macrophages release pro-inflammatory cytokines (blue dots) and trigger chronic tissue inflammation. Monocytes (yellow cells) are permanently attracted and migrate into periprosthetic tissue. Monocytes differentiate into macrophages, which are then getting activated by material surface promoting the chronic tissue inflammation. During chronic tissue inflammation, the released pro-inflammatory cytokines and activated macrophages themselves activate osteoclasts (orange cells), which then cause bone resorption and periprosthetic osteolysis. Furthermore, chronic inflammation results in the generation of fibrotic tissue (pink tissue, with pink dots), thus replacing functional periprosthetic soft tissue with fibrotic tissue. Aseptic chronic inflammation with implant-triggered tissue inflammation, osteolysis and fibrotic tissue formation is associated with the development of aseptic implant loosening.
During chronic low-grade infection, macrophages in periprosthetic tissue (dark red spiky-shaped cells) are constantly activated by the implant material surface and release pro-inflammatory cytokines (blue dots). Monocytes (yellow cells) permanently migrate into periprosthetic tissue and differentiate into macrophages. The chronic and compromised tissue inflammation favors the migration of bacteria into periprosthetic tissue, additionally fueling tissue inflammation. The compromised inflammatory response in the tissue is not able to clear bacteria, which then allows them to attach to the implant surface. Once attached to the implant surface, bacteria form a biofilm, which makes them resistant to immune cells and to antibiotic treatment. Activated macrophages and pro-inflammatory cytokines furthermore activate osteoclasts, which then start to resorb bone, resulting in periprosthetic osteolysis. Additionally, bacteria from biofilm release acidic factors (green dots), which cause bone demineralization. Chronic low-grade infection with chronic tissue inflammation, bacteria migration, biofilm formation, osteolysis and bone demineralization is associated with the development of septic implant loosening.
Fig. 2: Pathogenesis of chronic inflammation with orthopedic implants. The pathogenesis of chronic inflammation with orthopedic implants is exemplified by a hip implant.
It is very important to distinguish between aseptic chronic inflammation and chronic low-grade infection.
This figure was created with BioRender.com, 2024.
Advanced age is often associated with increased levels of several inflammatory molecules. The chronic, aseptic, low-grade inflammation occurring in older people is known as “inflammaging”.
Causes include senescence of cells and of the immune system, with increased circulating cell debris such as mitochondrial DNA associated with mitochondrial dysfunction, accumulation of pro-coagulation factors, free radicals, and reactive oxygen species (ROS), but also the increase in visceral body fat and the disruption of the gut microbiome. All these factors may lead to a chronic stimulation of the innate immune system with continuous release of proinflammatory molecules. Inflammaging is a risk factor for age-related morbidity, including cardiovascular diseases and mortality.6
Adipose tissue is now recognized as an endocrine organ, with adipocytes secreting metabolically active mediators called adipokines and other inflammatory cytokines and chemokines when stimulated by the excess of macronutrients, especially carbohydrates and fat. Several studies have also demonstrated a predominance of proinflammatory M1 macrophages in the fat tissue of obese people in contrast with the dominance of anti-inflammatory M2 macrophages in non-obese people.7 Concomitantly, the production of the hormone adiponectin by the adipocytes is reduced. Adiponectin plays an important role in lipid metabolism and insulin sensitivity and is also involved in immune responses and inflammation. Low levels of adiponectin levels are a significant predictor of cardiovascular mortality and have been associated with type 2 diabetes, cancer, stroke, and metabolic abnormalities. Reduced adiponectin levels in combination with the elevated secretion of pro-inflammatory molecules such as IL-6 from adipose cells, may lead to chronic inflammation (also called “metaflammation”) the metabolic syndrome associated with obesity (including insulin resistance, type 2 diabetes, coagulation, and cardiovascular disorders) and atherosclerosis.8 Obesity also has a detrimental effect on cartilage leading to osteoarthritis, in both weight-bearing joints and non-weight bearing articulations. Adipokines, including adiponectin and leptin, are important downregulators of inflammatory responses in cartilage, while other catabolic cytokines may inhibit the synthesis of proteoglycans and collagen type II, inducing cartilage degradation and bone resorption. Degradation products will elicit new inflammatory reactions, thus perpetuating the inflammatory process.9
Chronic inflammatory diseases have been associated with an unbalanced diet, rich in saturated fat and carbohydrates. As explained, increased intake of macronutrients may lead to higher production of pro-inflammatory molecules by the adipocytes.10 Additionally, unhealthy diets are often high in toxic contaminants (e.g., in thermally processed foods) and low in antioxidants (found in fruit, vegetables, and tea), which protect the cells from increased oxidative stress. Finally, the diet may have an impact on the composition and metabolism of the gut bacteria, the so-called microbiome. The gut microbiome consists of a diversity of microorganisms and performs important functions related not only to digestion and metabolization but to immune modulation. Gut microbiome dysbiosis, i.e. a disturbance in the composition and the ratio of microbial species, may cause breaches in the intestinal barrier, letting potentially harmful components into systemic circulation, thus stirring up an immune inflammatory response that may become chronic.11 The importance of diet cannot be underestimated as it is demonstrated to be the number- one risk factor in death and disability-adjusted life statistics.12 Diets high in fruits, vegetables, and fibers reduce inflammation and have a positive effect on global health and longevity.
Cigarette smoking is associated with chronic lung and cardiovascular disease, stroke, and cancer but is also generally recognized as a major risk factor for chronic inflammation.13 Toxins in cigarette smoke activate the secretion of proinflammatory molecules from mucosal cells in the oral cavity and the airways thereby inducing and sustaining inflammation. Cigarette smoke also contains trace amounts of bacterial lipopolysaccharides and other components triggering the immune response and leading to chronic inflammation.14 Blood samples of smokers have significantly higher levels of CRP, IL-6 and other inflammatory biomarkers.15 On the other hand, some elements of cigarette smoke may suppress the innate and adaptive defense against bacteria and neoplastic cells, thus increasing the risk of infection and cancer.
In large amounts, alcohol and its metabolites affect the liver, cause intestinal inflammation, alter the composition of the intestinal microbiome, impair its function, and damage the intestinal mucosal barrier. This leads to an additional inflammatory response, creating a vicious circle of chronic inflammation.16 Toxins such as gut microbiome-derived lipopolysaccharide (LPS) may also enter systemic circulation through breaches in the intestinal linings, causing inflammatory reactions and eventually irreversible organ damage.17
Long-term exposure to even low doses of chemical pollutants such as heavy metals, industrial chemicals, pesticides, food additives, or microplastics may lead to accumulation in the body and induce oxidative stress associated with chronic inflammation and cell and organ damage, as well as an impaired immune defense against microbial pathogens. Studies have shown that cocktails of pollutants are associated with an increase in systemic pro-inflammatory cytokines and activation of immune cells.18
Sedentarism and physical inactivity may lead to abdominal adiposity and visceral fat accumulation, which is associated with chronic systemic inflammation as described above.19 This lifestyle is also related to chronic inflammation independent of obesity. Researchers hypothesize that muscle disuse caused by inactivity disturbs the release of myokines from skeletal muscle, affecting immune regulation and promoting a pro-inflammatory pathway.20
Studies have demonstrated that stress activates neuroinflammatory responses in the brain. As stress activates the hypothalamo-pituitary-adrenal (HPA) axis, immune responses are normally suppressed through the secretion of glucocorticosteroids.21 Glucocorticosteroids have also been shown to activate the innate immune pathways to address danger signals. Prolonged and intense stress may thus overstimulate the immune system and lead to elevated pro-inflammatory cytokines, and accumulation of peripheral monocytes and macrophages in the brain and peripherally, causing chronic inflammation.22 The effect of stress on chronic inflammation is still under investigation.
Sleep disorders and irregular sleep schedules have been associated with a greater risk of inflammatory cytokine release and chronic inflammation. It is hypothesized that sleep disorders are correlated with other types of stress and with alterations of the circadian rhythm (e.g., in workers with night shifts) and the release of glucocorticosteroids.23
The genetic determinants of chronic inflammation have not been elucidated; but, for some chronic inflammatory diseases such as Crohn’s disease and diabetes type 1, shared genome loci have been identified.24 Two extensive genome-wide association studies have identified 58 loci for chronic inflammation related to CRP secretion.25
The relationship between gender and inflammation is well-known. Females are more often affected by autoimmune diseases but have fewer infections and more circulating antibodies. These findings may be associated with genes located on the X chromosome which are related to the immune system and may be overexpressed in females compared to males.26 On the other hand, there are relevant gender differences in oxidative stress mechanisms. In males, higher levels of ROS and other inflammatory markers have been associated with more oxidative cell damage and higher basal inflammation, possibly even accounting for higher mortality in comparison with females whose antioxidant mechanism and specific immune responses seem more efficient.27,28
In addition to gender differences, research has demonstrated that sex hormones like testosterone and estrogen may suppress the production and secretion of pro-inflammatory markers. Decreased production of sex hormones (e.g., in postmenopausal women) is often associated with the onset of inflammatory disorders, while maintaining sex hormone levels reduces the risk of several inflammatory diseases.29
Allergic disease is one of the most common chronic health disorders, affecting about 30% of the world’s population. People with a family history of allergies are at risk of developing allergic disease. In allergic people, exposure to otherwise harmless substances (called antigens or allergens) may elicit hypersensitivity responses, mediated by antibodies, immune complexes, or delayed lymphocytic cellular responses attacking the antigen. These types of adaptive immunity responses have been classified in four hypersensitivity classes (Type I-IV Gell and Cooms classification) and may result in chronic inflammation in cases of persistent or repetitive exposure to the allergens. About 4,000 different substances have been identified as potential allergens. Hypersensitivity to metals, including contact dermatitis, constitutes one of the prevalent forms of allergy. Sensitization to allergenic metals (about 45 of the 92 metal elements)30 may generate any of the four types of hypersensitivity responses, depending on the metal and the route of entry into the body. In addition to hypersensitivity reactions, metals may be immunotoxins and lead to the development of local inflammatory reactions, such as the adverse local tissue reactions (ALTR) associated with excessive metal wear from orthopedic implants,31 which usually involve innate immune mechanisms including the recruitment of macrophages rather than lymphocytes.32
Chronic inflammation in the context of joint replacement
Since joint replacements are subject to repetitive use, loading, and weight-bearing, the generation of wear products is inevitable. Materials used in orthopedic surgery, and more specifically in arthroplasty (including different metals, polymers, ceramics, and bone cements), will produce particulate debris and in some cases metal ions and corrosion products.33
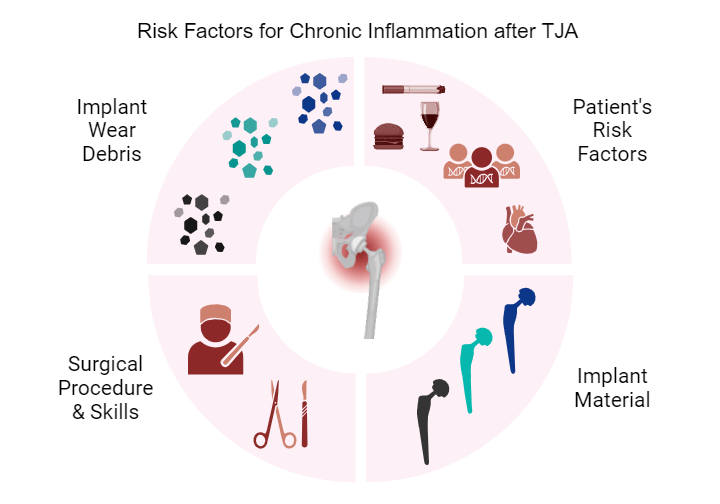
All these byproducts activate the innate immune system and even the adaptive immune system in patients with hypersensitivity to certain materials, usually metals. The development of chronic inflammation in the tissues surrounding a joint implant is multifaceted and may be connected to the implant material and its wear products, to the surgery, and/or to patient-related risk factors. As described above, the role of patient-related risk factors in the outcome of a joint replacement cannot be underestimated and is receiving more attention in the fields of personalized medicine and personalized arthroplasty.34 However, the importance of the implant material and the surgical technique and accuracy must be taken into account. Regarding the implant material, extensive fundamental and clinical studies have identified different volumetric wear rates and wear debris in association with specific bearing couples in total joint replacements.35 Determinants of the bioreactivity of wear debris (i.e., the potential immunological reaction to particles, metal ions, and even metal corrosion products) include the quantity, size, morphology, and chemical composition of the particles. These factors are related to the wear mechanism, namely the severity, rate, mode, and source of the wear.36 Large conventional polyethylene wear particles have been associated with extensive foreign body reactions and massive macrophage recruitment, as well as osteoclast activation causing osteolysis.37 In cases of excessive wear of metal-on-metal hip implants, the smaller metal particles have led to cases of ALTR.38 In addition, the chemical composition of the particles and the occurrence of metal ions and corrosion products may cause additional toxic reactions featuring cell death and tissue necrosis.39 Newer material combinations for articulating surfaces, such as cross-linked polyethylene and zirconia-toughened alumina, have exhibited much less generation of wear particles, resulting in lower implant failure and revision rates.40
Regarding the surgical factor, several aspects need to be considered. Firstly, every surgical intervention causes a tissue injury and will inevitably elicit an inflammatory reaction. In normal circumstances this inflammatory response is moderate and resolves after 2-14 days. In some cases, however, the surgical procedure triggers systemic inflammation and/or chronic postoperative pain.41 Secondly, in arthroplasty, surgical skills and accuracy of implant positioning are paramount to preventing articulating components’ dislocation, impinging, or exhibiting excessive wear. The latter occurs in hip resurfacing in cases of steep acetabular cup positioning, leading to edge-loading on the femoral head, which in turn inevitably leads to higher wear-generating particles and other wear debris such as metal ions from metal-on-metal articulations.42
In cases of high wear with an overload of particulate debris following component malpositioning, the immune system will not be able to eliminate the causative agents, leading to a continuous cytokine activation and recruitment of mononuclear leucocytes, eventually resulting in chronic inflammation, osteolysis, fibrosis, and granulomatosis. This chronic inflammation is associated with clinical symptoms such as pain and dysfunction and possibly prosthetic failure. Additionally, dormant bacterial biofilms on implant and particle surfaces may be activated in the compromised environment and lead to a low-grade infection, complicating, and perpetuating the chronic inflammation. The differences in diagnosis between aseptic chronic inflammation and low-grade infection is also important regarding the choice of therapeutic interventions. While extensive osteolysis, prosthetic loosening, or clear-cut periprosthetic infections necessitate revision surgery, non-surgical therapeutic interventions with osteogenic, cellular, and immunotherapeutic agents may be used in the future to disrupt the inflammatory vicious cycle and salvage an otherwise well-functioning implant.43
Conclusion
From a preventive point of view, several factors are paramount: careful surgical technique and implant positioning; preference of materials exhibiting low wear, low immunogenicity, and low bacterial adherence; and potential risk-associated host factors. Evidently, patient- risk factors are often interrelated. Arthroplasty patient populations’ risk factors for chronic inflammation can include advanced age, obesity, and low physical activity. They are more susceptible to the development of adverse inflammatory effects after the implantation of a prosthetic joint. During the preoperative and postoperative period, patients should be encouraged and supported to adopt a healthy lifestyle including a balanced diet, weight loss if necessary, and physical exercise. The good news is that the arthroplasty itself will enhance the patient’s quality of life and enable them to establish habits that promote a more active lifestyle. Successful hip and knee replacements can be life-changing interventions, associated with less overall morbidity and lower mortality.44,45
References
1. Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9(6):7204-7218. doi:10.18632/oncotarget.23208.
2. Pahwa R, Goyal A, Jialal I. Chronic Inflammation. StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2024.
3. Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 2018;4(12):e01067. doi:10.1016/j.heliyon.2018.e01067.
4. Versari A. Nuclear Medicine Imaging in Chronic Inflammatory Diseases. Radionuclide Imaging of Infection and Inflammation. Springer; 2013.
5. Liu CH, Abrams ND, Carrick DM, et al. Imaging inflammation and its resolution in health and disease: current status, clinical needs, challenges, and opportunities. Faseb j. 2019;33(12):13085-13097. doi:10.1096/fj.201902024.
6. Sanada F, Taniyama Y, Muratsu J, et al. Source of chronic inflammation in aging. Front Cardiovasc Med. 2018;5:12. doi:10.3389/fcvm.2018.00012.
7. Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851-863. doi:10.5114/aoms.2016.58928.
8. Khanna D, Khanna S, Khanna P, Kahar P, Patel BM. Obesity: a chronic low-grade inflammation and its markers. Cureus. 2022;14(2):e22711. doi:10.7759/cureus.22711.
9. Wang T, He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38-50. doi:10.1016/j.cytogfr.2018.10.002.
10. Margină D, Ungurianu A, Purdel C, et al. Chronic inflammation in the context of everyday life: dietary changes as mitigating factors. Int J Environ Res Public Health. 2020;17(11):4135. doi:10.3390/ijerph17114135.
11. Wagenaar CA, van de Put M, Bisschops M, et al. The effect of dietary interventions on chronic inflammatory diseases in relation to the microbiome: a systematic review. Nutrients. 2021;13(9):3208. doi:10.3390/nu13093208.
12. Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982-3021. doi:10.1016/j.jacc.2020.11.010.
13. Bakhru A, Erlinger TP. Smoking cessation and cardiovascular disease risk factors: results from the Third National Health and Nutrition Examination Survey. PLoS Med. 2005;2(6):e160. doi:10.1371/journal.pmed.0020160.
14. Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res. 2012;91(2):142-149. doi:10.1177/0022034511421200.
15. Elisia I, Lam V, Cho B, et al. The effect of smoking on chronic inflammation, immune function and blood cell composition. Sci Rep. 2020;10(1):19480. doi:10.1038/s41598-020-76556-7.
16. Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol. 2010;16(11):1304-1313. doi:10.3748/wjg.v16.i11.1304.
17. Bishehsari F, Magno E, Swanson G, et al. Alcohol and gut-derived inflammation. Alcohol Res. 2017;38(2):163-171.
18. Liu Y, Zhang Z, Han D, Zhao Y, Yan X, Cui S. Association between environmental chemicals co-exposure and peripheral blood immune-inflammatory indicators. Front Public Health. 2022;10:980987. doi:10.3389/fpubh.2022.980987.
19. Burini RC, Anderson E, Durstine JL, Carson JA. Inflammation, physical activity, and chronic disease: an evolutionary perspective. Sports Med Health Sci. 2020;2(1):1-6. doi:10.1016/j.smhs.2020.03.004.
20. Nelke C, Dziewas R, Minnerup J, Meuth SG, Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. 2019;49:381-388. doi:10.1016/j.ebiom.2019.10.034.
21. Liu YZ, Wang YX, Jiang CL. Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci. 2017;11:316. doi:10.3389/fnhum.2017.00316.
22. Kim IB, Lee JH, Park SC. The relationship between stress, inflammation, and depression. Biomedicines. 2022;10(8):1929. doi:10.3390/biomedicines10081929.
23. Ditmer M, Gabryelska A, Turkiewicz S, Białasiewicz P, Małecka-Wojciesko E, Sochal M. Sleep problems in chronic inflammatory diseases: prevalence, treatment, and new perspectives: a narrative review. J Clin Med. 2021;11(1):67. doi:10.3390/jcm11010067.
24. Heap GA, van Heel DA. The genetics of chronic inflammatory diseases. Hum Mol Genet. 2009;18(R1):R101-R106. doi:10.1093/hmg/ddp001.
25. Ligthart S, Vaez A, Võsa U, et al. Genome analyses of >200,000 individuals identify 58 loci for chronic inflammation and highlight pathways that link inflammation and complex disorders. Am J Hum Genet. 2018;103(5):691-706. doi:10.1016/j.ajhg.2018.09.009.
26. Trabace L, Roviezzo F, Rossi A. Editorial: sex differences in inflammatory diseases. Front Pharmacol. 2022;13:962869. doi:10.3389/fphar.2022.962869.
27. Casimir GJ, Duchateau J. Gender differences in inflammatory processes could explain poorer prognosis for males. J Clin Microbiol. 2011;49(1):478; author reply 478-9. doi:10.1128/jcm.02096-10.
28. Martínez de Toda I, González-Sánchez M, Díaz-Del Cerro E, Valera G, Carracedo J, Guerra-Pérez N. Sex differences in markers of oxidation and inflammation. Implications for ageing. Mech Ageing Dev. 2023;211:111797. doi:10.1016/j.mad.2023.111797.
29. Wei C, Zhang W, Chen J, et al. Systematic analysis between inflammation-related index and sex hormones in American adults: cross-sectional research based NHANES 2013-2016. Front Immunol. 2023;14:1175764. doi:10.3389/fimmu.2023.1175764.
30. Thyssen JP, Menné T. Metal allergy--a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 2010;23(2):309-318. doi:10.1021/tx9002726.
31. Van Der Straeten C, Grammatopoulos G, Gill HS, Calistri A, Campbell P, De Smet KA. The 2012 Otto Aufranc Award: the interpretation of metal ion levels in unilateral and bilateral hip resurfacing. Clin Orthop Relat Res. 2013;471(2):377-385. doi:10.1007/s11999-012-2526-x.
32. Roach K, Roberts J. A comprehensive summary of disease variants implicated in metal allergy. J Toxicol Environ Health B Crit Rev. 2022;25(6):279-341. doi:10.1080/10937404.2022.2104981.
33. Nine MJ, Choudhury D, Hee AC, Mootanah R, Osman NAA. Wear debris characterization and corresponding biological response: artificial hip and knee joints. Materials (Basel). 2014;7(2):980-1016. doi:10.3390/ma7020980.
34. Vendittoli PA, Riviere C, Hirschmann MT, Bini S. Why personalized surgery is the future of hip and knee arthroplasty: a statement from the Personalized Arthroplasty Society. EFORT Open Rev. 2023;8(12):874-882. doi:10.1530/eor-22-0096.
35. Sonntag R, Reinders J, Kretzer JP. Bio-Tribological Demands. In: Sonntag R, Kretzer JP, eds. Materials for Total Joint Arthroplasty. World Scientific; 2016:1-13.
36. Billi F, Benya P, Kavanaugh A, Adams J, Ebramzadeh E, McKellop H. The John Charnley Award: an accurate and sensitive method to separate, display, and characterize wear debris: part 1: polyethylene particles. Clin Orthop Relat Res. 2012;470(2):329-338. doi:10.1007/s11999-011-2057-x.
37. Yin Z, Gong G, Liu X, Yin J. Mechanism of regulating macrophages/osteoclasts in attenuating wear particle-induced aseptic osteolysis. Front Immunol. 2023;14:1274679. doi:10.3389/fimmu.2023.1274679.
38. Eltit F, Wang Q, Wang R. Mechanisms of adverse local tissue reactions to hip implants. Front Bioeng Biotechnol. 2019;7(176):eCollection 2019. doi:10.3389/fbioe.2019.00176.
39. Billi F, Campbell P. Nanotoxicology of metal wear particles in total joint arthroplasty: a review of current concepts. J Appl Biomater Biomech. 2010;8(1):1-6.
40. Smith P, Gill D, McAuliffe M, et al. Hip, Knee and Shoulder Arthroplasty: 2023 Annual Report Australian Orthopaedic Association National Joint Replacement Registry. 2023.
41. Thapa P, Euasobhon P. Chronic postsurgical pain: current evidence for prevention and management. Korean J Pain. 2018;31(3):155-173. doi:10.3344/kjp.2018.31.3.155.
42. Amstutz HC, Le Duff MJ, Johnson AJ. Socket position determines hip resurfacing 10-year survivorship. Clin Orthop Relat Res. 2012;470(11):3127-3133. doi:10.1007/s11999-012-2347-y.
43. Goodman SB, Gallo J, Gibon E, Takagi M. Diagnosis and management of implant debris-associated inflammation. Expert Rev Med Devices. 2020;17(1):41-56. doi:10.1080/17434440.2020.1702024.
44. Cnudde P, Rolfson O, Timperley AJ, et al. Do patients live longer after THA and is the relative survival diagnosis-specific? Clin Orthop Relat Res. 2018;476(6):1166-1175. doi:10.1007/s11999.0000000000000097.
45. Palazzuolo M, Antoniadis A, Mahlouly J, Wegrzyn J. Total knee arthroplasty improves the quality-adjusted life years in patients who exceeded their estimated life expectancy. Int Orthop. 2021;45(3):635-641. doi:10.1007/s00264-020-04917-y.