While both deal with inserting foreign bodies into bone, there are many fundamental differences between arthroplasty and dental implantology. However, with peri-implant infection they have one major complication in common. Bacteria strains and biofilm dynamics may differ, but the basic processes are the same. The studies show that the role of implant material regarding infection is similar in both fields.
Nowadays, implants used in joint replacement and in oral implantology are common devices used to treat different diseases and impairments with different aims. Arthroplasty intends to reduce pain and to restore function; oral implantology is meant to replace teeth lost because of injury, periodontal disease or agenesia. The fight against bacteria and infection is one thing orthopedic surgeons and implantologists have in common.
Differences between joint and dental implants
Periprosthetic joint infection, mucositis and peri-implantitis all lead to the failure of the implant but differ in several aspects:
- Whereas periprosthetic joint infection (PJI) can lead to a significant incidence of mortality, an infection of the oral implant is not life-threatening. Peri-implantitis is rather uncomfortable for the patients and costly due to the necessity of implant replacement.
- Infection is more frequent in dentistry than in orthopedics. The 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions extensively reviewed the incidence and the comorbidity of oral mucositis and peri-implantitis concluding that both conditions are very frequent especially in patients with a medical history of periodontitis.1
- In joint replacement, periprosthetic joint infection is involved in 14-29% of the implant failures.2 While artificial joints do not pass the sterile barrier, dental implants are transmucosal devices. The part below the soft tissue sealing must be sterile while the part above it naturally hosts the oral microbiota. Thanks to the soft tissue sealing at the implant’s neck microbiota does not invade the bone below.
- Microbial biofilm naturally adheres to oral implant surfaces coronally to the epithelium attachment. The infection disease is not due to the presence of bacteria itself but to an unbalanced microbiota ecosystem with prevalence of Gram-negative anaerobic bacteria that trigger and boost inflammation and then invade the tissues. Bacteria adhesion and biofilm formation differ between biomaterial surfaces used in orthopedic implants. Significantly less biofilm is formed on ceramic surfaces compared to polyethylene and metal.3
- The presence of an orthopedic implant reduces the bacterial concentration required to induce infection by 100,000 times2, since bacteria can survive in the periprosthetic environment by adhering to the implant. While in arthroplasty periprosthetic joint infections are due to a small number of bacteria species, mainly nosocomial, in oral implantology the infection is caused by a complex biofilm composed of many different species.4
Biofilm formation and development
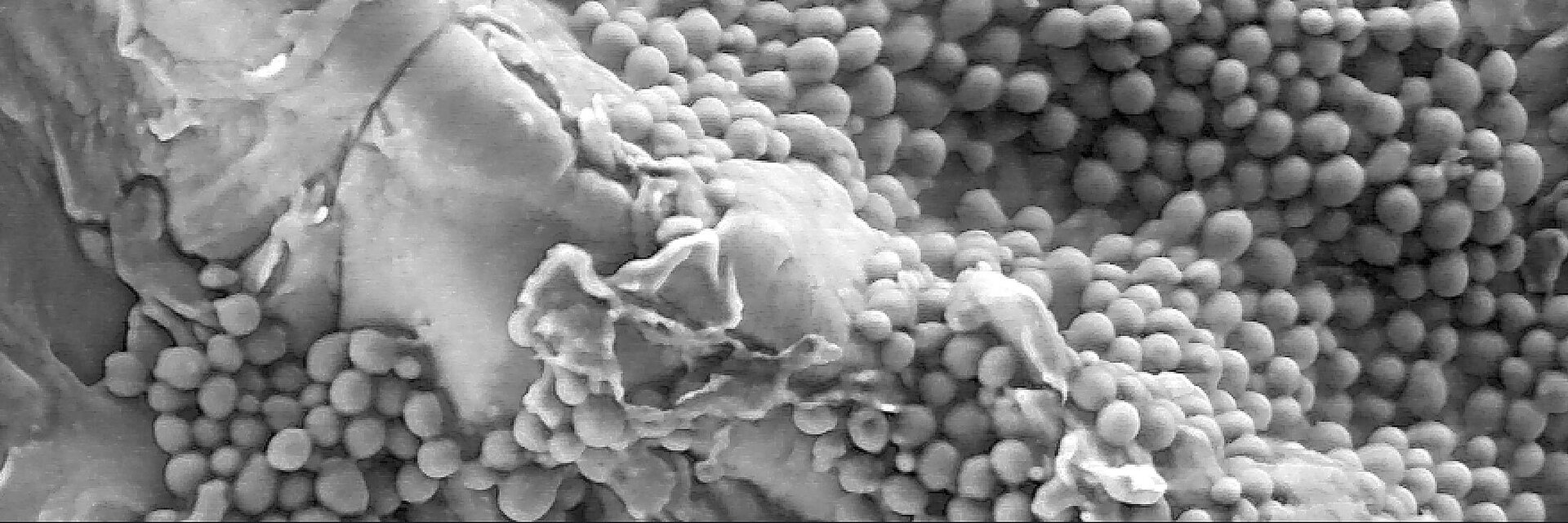
The formation and composition of a biofilm strongly depends on the substrate surface on which it grows. The surface properties play a more important role in dentistry than in orthopedics, even if the formation of the biofilm is very similar (Fig. 1). In the oral cavity, the formation starts when planktonic bacteria coming from saliva approach the implant surface and initially interact with it by electrostatic forces. Once adherent, bacteria improve the adhesion on the surface via receptor-ligand interaction. They proliferate and secrete different kinds of macromolecules, principally polysaccharides and glycolipids, known as extracellular polymeric substances, which embed and protect the newly formed bacteria community. In joint replacement, biofilm formation begins when planktonic bacteria, mostly coming from the surgical incision site or from independent infection sources, escape immunological surveillance and adhere to the implant surface.5 In peri-implant joint infection, the bacteria community is formed by few species. The most common bacteria involved are nosocomial strains, often antibiotic-resistant, including the so-called ESKAPE bacteria (S. aureus, S.epidermidis, Enterococcus faecium, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.).6
In the oral cavity, the composition of the biofilm which grows on natural surfaces and on devices is made up by commensals and changes from the early to the late stages. In the first phases aerobic and Gram-positive commensals bacteria are predominant; during maturation the community is enriched by pathobiontic Gram-negative anaerobic bacteria. Therefore, the easy strategy to maintain the oral peri-implant tissue in a healthy condition is to keep the colonization of the implant surfaces at the level of the early stages rather than to avoid any colonization.
In case of dental implants, daily hygiene procedures contribute to remove the biofilm mechanically. In arthroplasty, apart from clinical protocol optimizations, the use and design of “low contamination devices” such as highly polished or modified metallic surfaces and ceramics could be an alternative strategy.
Biofilm viability of different materials
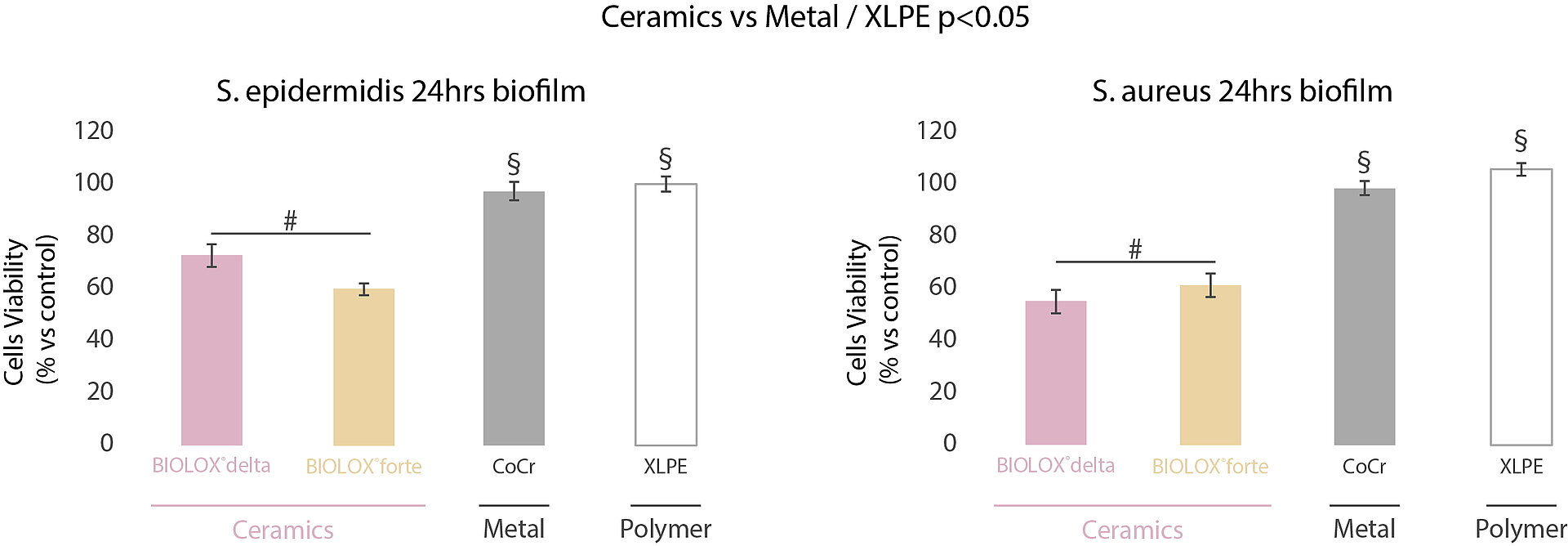
In a multicentric and interdisciplinary study7, we compared the bacteria adhesion mechanisms on CoCr, XLPE, alumina (BIOLOX®forte) and zirconia-toughened alumina (ZTA) ceramic (BIOLOX®delta). The MTT evaluation of the biofilm formed on the surface of the investigated orthopedic materials indicated the highest biofilm viability for XLPE and CoCrMo surfaces after 24 hours (Fig. 2).
In comparison, ceramic samples showed reduced bacterial adhesion and slower biofilm development with both bacteria strains tested (S. aureus and S. epidermidis). In-vitro and in-vivo, ceramic materials have shown their ability to accumulate thinner biofilm in the short term compared to metal and polymers (Fig. 3).8 These observations suggest that ceramic surfaces may contribute to the prevention of prosthetic joint infection and oral peri-implant mucositis (if associated with proper daily biofilm removal). The arthroplasty registries demonstrated the lower risk of revision for deep infection in hip replacement at long term with ceramic bearings. In contrast, there is no clear long-term information available in dental implantology, mainly due to the limited available literature and the absence of broadbased national registers. The risk factors for the incidence of infection are manyfold and – just as in arthroplasty – only observational studies including the analysis of granular and robust registry data will allow to detect the independent factors associated with implant infection in dental implantology, too.
References
1. Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89 Suppl 1:S313-S318. doi:10.1002/JPER.17-0739.
2. Song Z, Borgwardt L, Høiby N, Wu H, Sørensen TS, Borgwardt A. Prosthesis infections after orthopedic joint replacement: the possible role of bacterial biofilms. Orthop Rev (Pavia). 2013;5(2):65-71. doi:10.4081/or.2013.e14.
3. Trampuz A, Maiolo EM, Winkler T, Perka C. Biofilm formation on ceramic, metal and polyethylene bearing components from hip joint replacement systems. Orthopaedic Proceedings. 2016;98-B (SUPP 10):80.
4. Gbejuade HO, Lovering AM, Webb JC. The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015;86(2):147-158. doi:10.3109/17453674.2014.966290.
5. Rimondini L, Della Valle C, Cochis A, Azzimonti B, Chiesa R. The biofilm formation onto implants and prosthetic materials may be contrasted using gallium (3+). Key Eng. Mater. 2013;587:315-320. doi:10.4028/www.scientific.net/ KEM.587.315.
6. Ryu DJ, Kang JS, Moon KH, Kim MK, Kwon DG. Clinical characteristics of methicillin-resistant Staphylococcus aureusiInfection for chronic periprosthetic hip and knee infection. Hip Pelvis. 2014;26(4):235-242. doi:10.5371/ hp.2014.26.4.235.
7. Sorrentino R, Cochis A, Azzimonti B, et al. Reduced bacterial adhesion on ceramics used for arthroplasty applications. J Eur Ceram Soc. 2018;38(3):963-970. doi:10.1016/j.jeurceramsoc.2017.10.008.
8. Rimondini L, Cerroni L, Carrassi A, Torricelli P. Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J Oral Maxillofac Implants. 2002;17:793-798.