Introduced to the market over 15 years ago, BIOLOX®delta ceramic hip implants have been widely used in primary total hip arthroplasty ever since. Their clinical performance is well documented in clinical studies and implant registries, with abundant evidence supporting their efficacy and safety. The collected data support the value of BIOLOX®delta as a reliable option in hip revision, too.
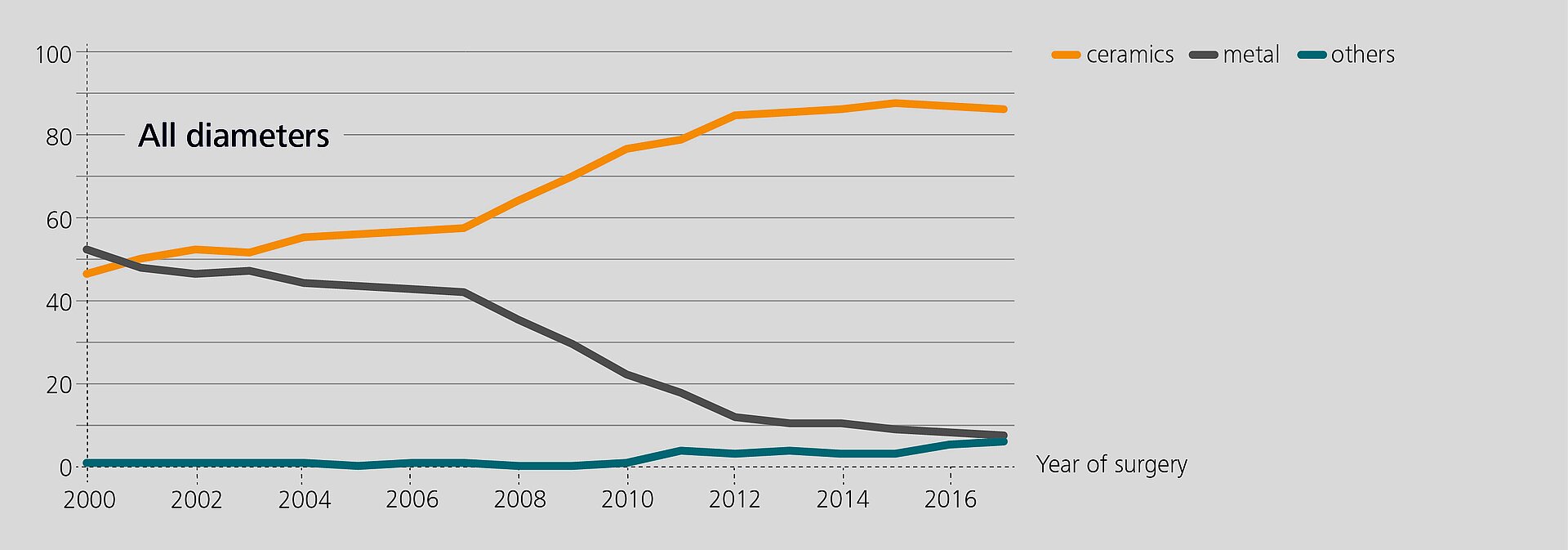
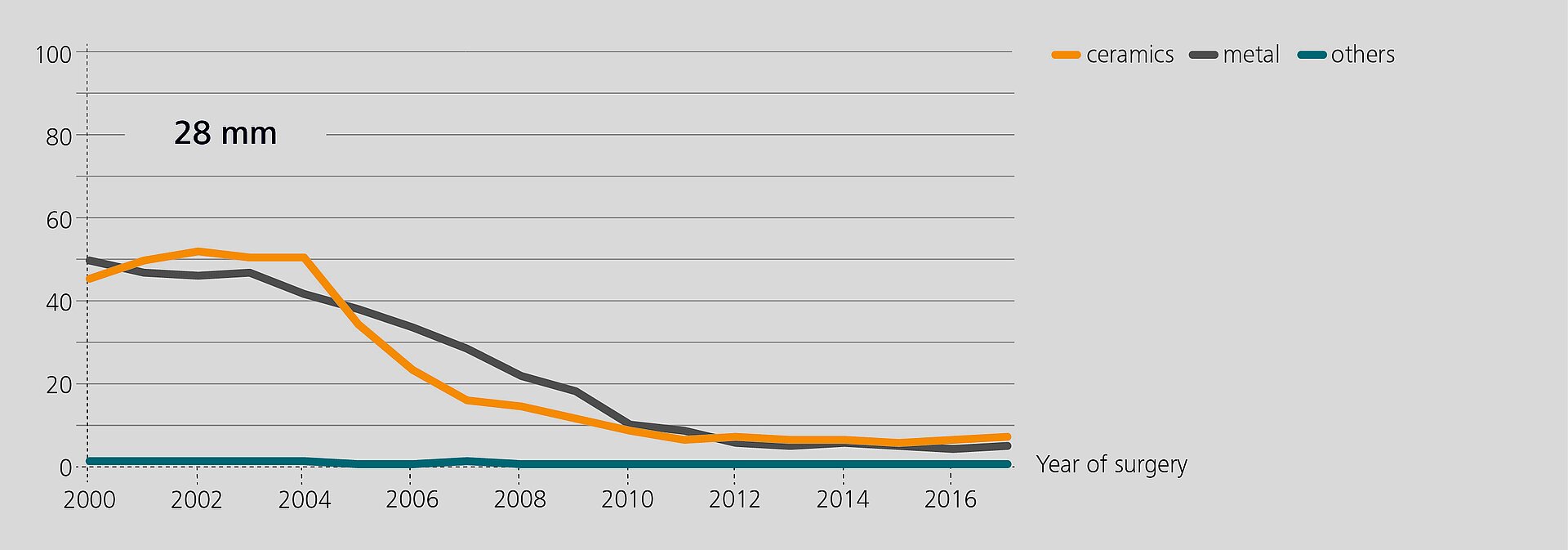
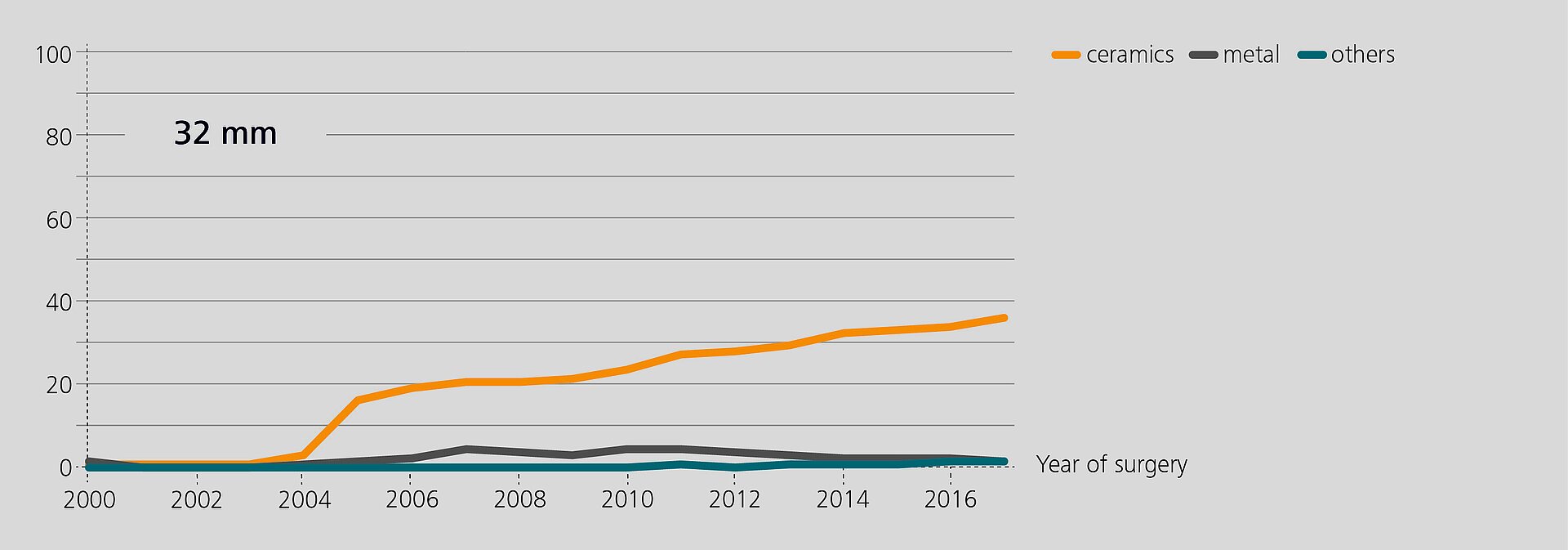
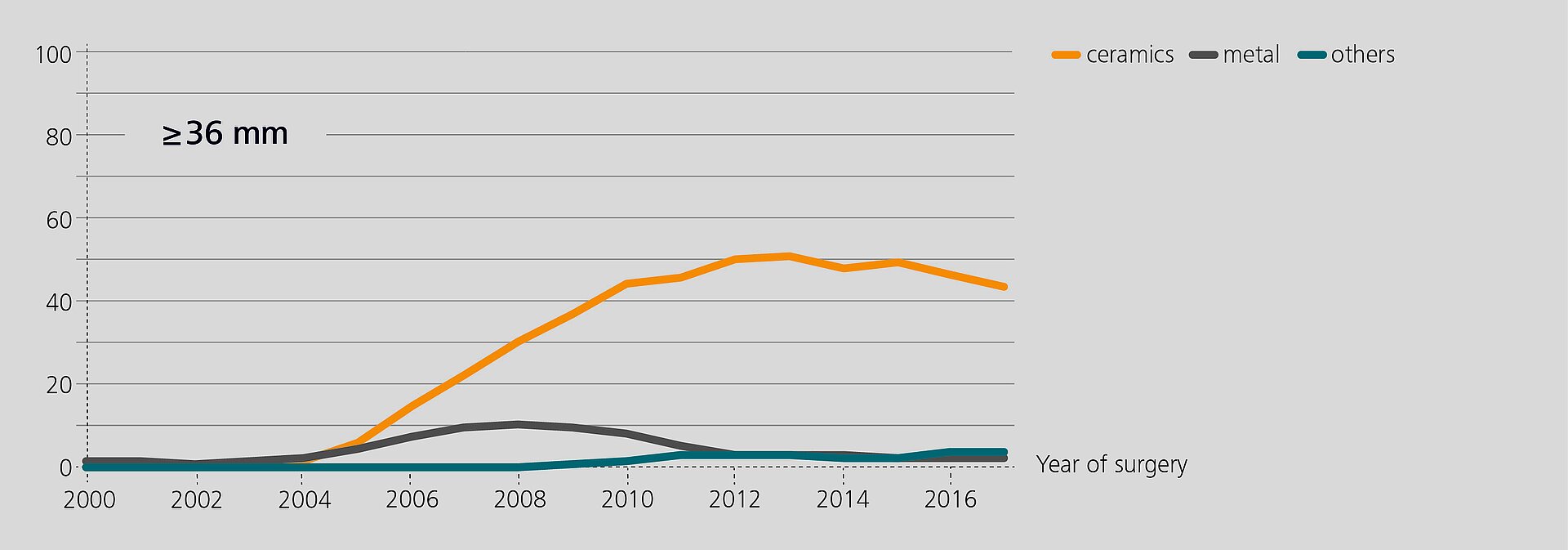
Researchers from the Rizzoli Orthopaedic Institute (Bologna, Italy) have recently analyzed the performance of BIOLOX®delta components in revision based on data from the regional Register of Orthopaedic Prosthetic Implantology (RIPO)1,2. In 2018, 98% of all hip, knee and shoulder arthroplasties performed in the Emilia-Romagna region were captured. The latest report (2000–2017) offers a documentation of 111,856 primary total hip replacements and of 16,432 hip revisions. Ceramic-on-Ceramic (CoC) was the bearing couple most commonly used in primary total hip arthroplasty (45.6%). In revision, 25.5% of the hip with a CoC bearing. According to the registry, alumina composite ceramics and XLPE have become the preferred bearing materials for index surgery. The survival rate of all primary hip procedures at 17 years is 89.1%3.
Clinical Performance at Mid-term Follow-up
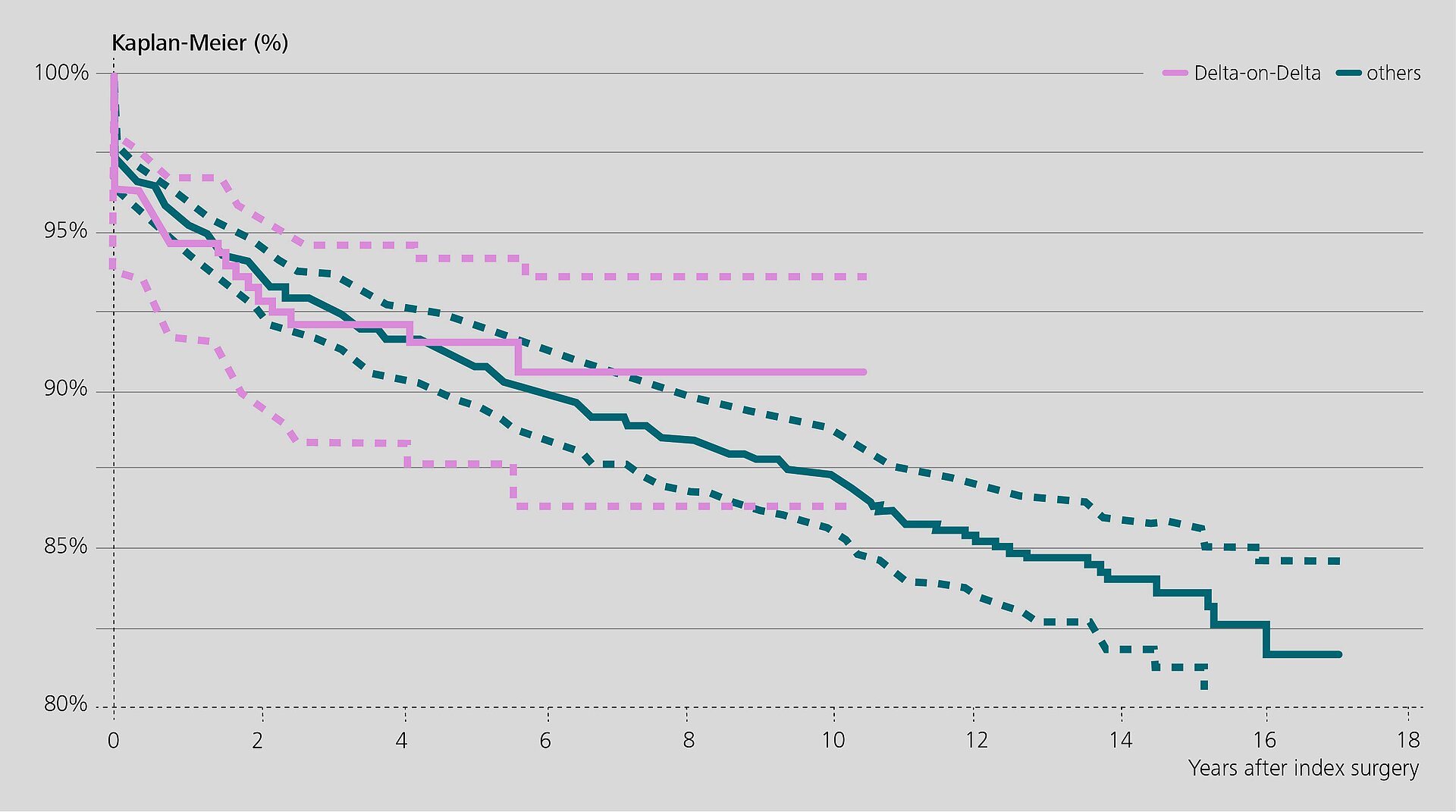
In one study1, the Rizzoli group analyzed RIPO data to evaluate the survival rate of 327 revision implants with BIOLOX®delta-on-BIOLOX®delta ceramic bearings (hereafter: Delta-on-Delta). The survival rate was 90.5% at 10 years (mean follow-up: 4.1 years). Twenty-three hips were re-revised and the reasons for failure were analyzed.
Recurrent dislocation was identified as the main reason for failure. Most of the re-revisions occurred within the first year after the original revision surgery. According to the authors, the prevalence of recurrent dislocations may be related to implant positioning or soft tissue quality / tension issues. Re-revisions for ceramic fracture or noise were not reported.
When compared to the control group with other bearing types, the researchers found a lower re-revision rate for implants with Delta-on-Delta. The ceramic composite bearings also showed a lower incidence of re-revision for aseptic and septic loosening. The authors concluded that revision procedures with Delta-on-Delta bearings show good and reliable clinical performance at mid-term follow-up.
These positive results are supported by a retrospective clinical study from Korea in 2018. Chang et al.4 evaluated the clinical and radiological outcomes of 47 patients (52 hips) treated with Delta-on-Delta in revision surgery with all components exchanged. None of the components required a re-revision at a mean follow-up of 7.3 years. The study reported no ceramic fractures or noise.
These results correspond to the outcome in a small case series (18 hips, 16 patients) by the already mentioned Rizzoli group. In this clinical study, Castagnini et al.5 report very good clinical results of highly porous titanium cups with Delta-on-Delta bearings with an adapter-sleeved head.
Sixteen patients with metal resurfacing cups were revised. They received isolated acetabular revision cups and a Delta-on-Delta bearings. All patients showed elevated metal ion concentrations at the time of surgery. The clinical outcome of the revisions was evaluated at a minimum follow-up of 5 years. None of the 16 patients had to be re-revised, no patient was lost to follow-up. The metal-ion level in both blood and urine continually decreased after revision.
In an earlier study from 2016, Plummer et al.6 report similar results in a small cohort of patients at short term follow-up: ALTR patients revised with a sleeved ceramic head had significantly decreased cobalt and chromium ion concentration in the blood compared to ALTR patients revised with a metal head. Based on these results, the authors suggested to retain a well-fixed femoral stem and to use sleeved ceramic heads in ALTR patients.
However, some surgeons raise concerns about the addition of a titanium sleeve between the stem and the ceramic head as potential trigger of fretting corrosion at the stem/sleeve interface.
Preuss et al.7 addressed this question and performed in-vitro fretting corrosion tests according to ASTM F1875. Titanium alloy, cobalt chrome and stainless steel stems were evaluated with a sleeved BIOLOX®OPTION head. The authors did not find “any sign of excessive surface deterioration or progressive degradation.” This was confirmed clinically by Eichler et al.8. The Canadian research group measured the cobalt, chrome and titanium ion levels in the whole blood of 36 primary THA patients treated with Delta-on-Delta with large-diameter (sleeved) heads (40mm–48mm) and a preassembled acetabular component. After 5-years follow-up, the ion levels were low and no significant changes were noticed over time. No signs of trunnionosis were detected.
An analysis of retrieved BIOLOX®OPTION sleeved heads at the Hospital of Special Surgery, New York, performed by Koch et al.9 also showed that BIOLOX®delta sleeved ceramic heads are safe in terms of fretting corrosion. An investigation of retrievals at Drexel University, Philadelphia (MacDonald et al.)10 also supports their use.
To Sleeve or Not to Sleeve Is a Matter of Risk
The international orthopedic community still seems to be divided on the necessity of placing a sleeve on a used taper although the implant manufacturers recommend this expressively.
Placing a BIOLOX®delta head on a retained stem without an adapter is a matter of risk mitigation. Stay on the safe side by using a sleeved femoral head.
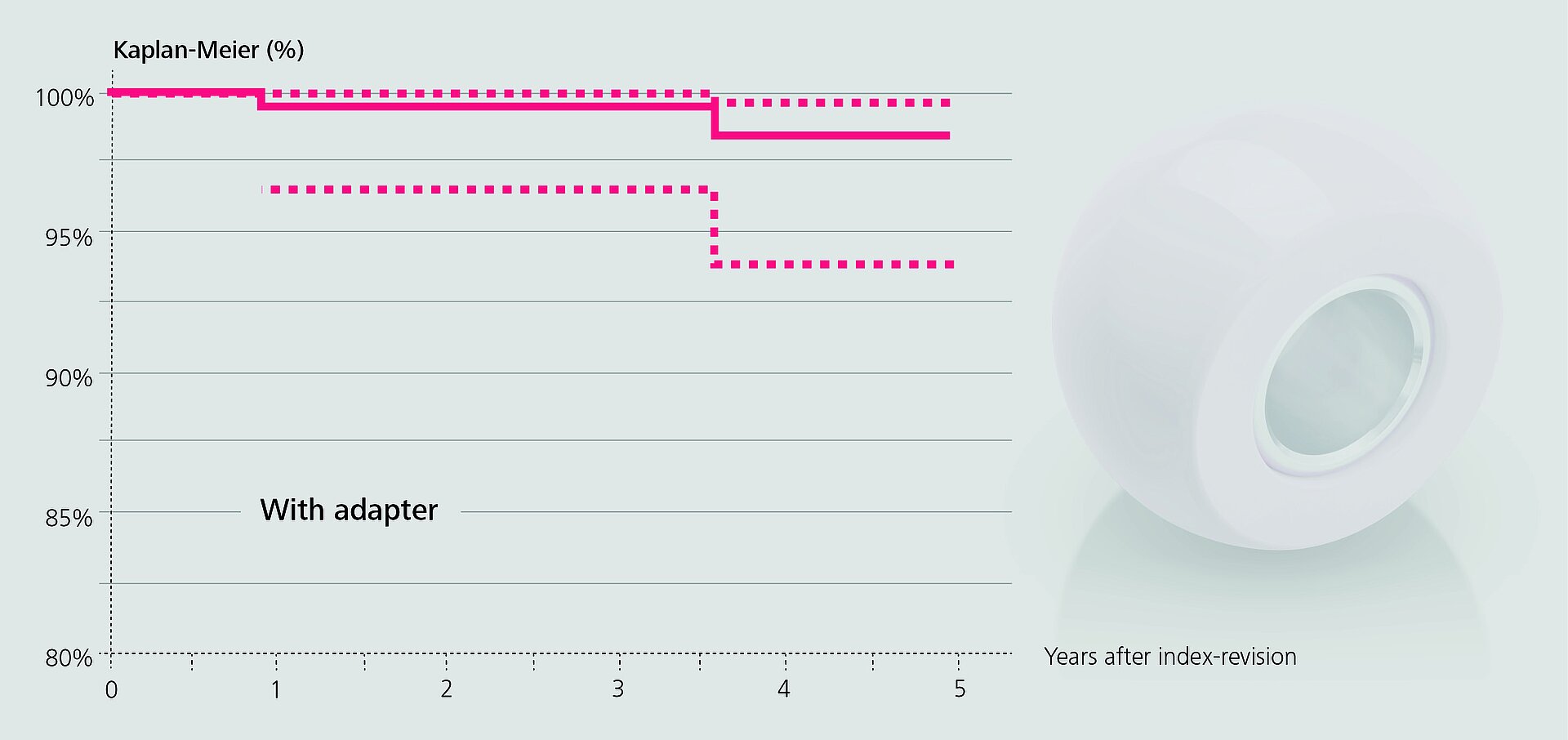
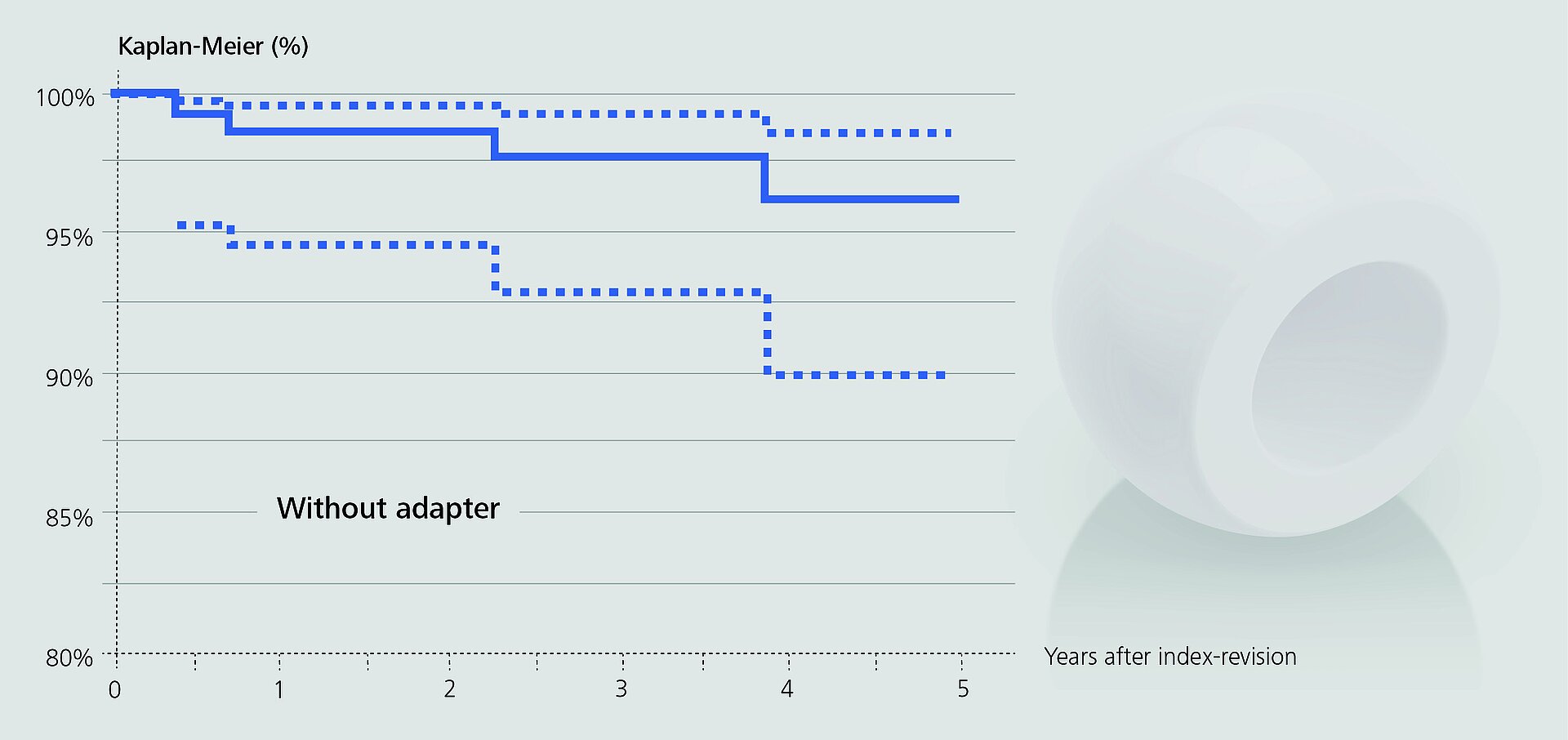
In an observational study based on RIPO data, Affatato et al.2 investigated the impact of adapter sleeves on re-revision rates of hip revision procedures with retained stems. Metal heads were mostly used without sleeves, about two thirds of the ceramic heads were implanted with sleeves.
In the remaining cases, the surgeons opted for a non-sleeved BIOLOX®delta head. The authors found that the use of adapter sleeves significantly reduced the rate of re-revisions when all head materials were included (98.4% vs. 95.2% survival rate). Since there were no fractures of non-sleeved BIOLOX®delta heads at mid-term follow-up, the authors concluded that the use of the sleeve may play a “negligible role” in this respect.
Kim et al.11 came to a similar conclusion. In a prospective clinical study, the Korean group implanted Delta-on-Delta bearings without using an adapter sleeve in 100 hip revisions. The revisions became necessary because of polyethylene wear and osteolysis with metal-on-polyethylene bearings. The tapers of the retained stems showed minimal to mild corrosion. The damage status of the taper was evaluated by three surgeons using a magnifying lens. Based on this visual inspection, they opted for non-sleeved heads.
After a mean follow-up of 12.8 years, no ceramic fractures were detected. Based on these results, the authors concluded that the use of adapter sleeves on stem tapers remaining in situ is not necessary.
Stay on the Safe Side
Even if these studies seem to support this option, implant manufacturers and CeramTec expressly caution surgeons to consider this decision carefully as it contravenes the Instructions for Use. The function of a sleeve is to recreate a pristine taper which is a prerequisite when engaging a new femoral ceramic head onto a slightly damaged stem taper.
The necessity of placing a sleeve has been recently addressed by Morlock's research group in Hamburg12. The authors demonstrated that small local metal elevations at the edge of a scratch or at a damaged surface area of the taper can potentially lead to early failure of non-sleeved BIOLOX®delta ceramic heads despite their high fracture strength. Adapter sleeves allow an even distribution of contact stresses between stem taper and head, compensating local taper damage. Since local taper damage cannot be totally excluded by visual inspection, a sleeve should always be used on stem tapers remaining in situ in order to guarantee optimal conditions for BIOLOX®delta heads.
CeramTec is committed to selecting and bringing to interested parties relevant articles on bioceramics related topics. The presented authors’ views and opinions are solely those of the authors of these publications. It is the focus and intent of CeraNews that CeramTec presents and comments on the authors’ views and opinions in a specific context. Such comments and editorials therefore solely express CeramTec’s views and opinions and not necessarily those of the quoted authors.
References
- Castagnini F, Bordini B, Tassinari E, Stea S, Ancarani C, Traina F. Delta-on-Delta ceramic bearing surfaces in revision hip arthroplasty. J Arthroplasty. 2019;34(9):2065-2071. doi:10.1016/j.arth.2019.04.068.
- Affatato S, Cosentino M, Castagnini F, Bordini B. Registry study on failure incidence in 1,127 revised hip implants with stem trunnion re-use after 10 years of follow-up: limited influence of an adapter sleeve. Acta Orthop. 2019;90(5):417- 420. doi:10.1080/17453674.2019.1618649.
- Castagnini F, Mariotti F, Tassinari E, Bordini B, Zuccheri F, Traina F. lsolated acetabular revisions of articular surface replacement (ASR) XL implants with highly porous titanium cups and Delta bearings. Hip Int. 2019:1120700019874442. doi:10.1177/1120700019874442.
- Report of R.I.P.O. Regional Register of Orthopaedic Prosthetic Implantology Overall Data Hip, Knee and Shoulder Arthroplasty in Emilia-Romagna Region (Italy) 2000-2017 Version 1 July 22, 2019. 2019:6-7.
- Chang JD, Kim IS, Mansukhani SA, Sharma V, Lee SS, Yoo JH. Midterm outcome of fourth-generation ceramic-on-ceramic bearing surfaces in revision total hip arthroplasty. J Orthop Surg (Hong Kong). 2018;26(2):2309499018783913. doi:10.1177/2309499018783913.
- Plummer DR, Berger RA, Paprosky WG, Sporer SM, Jacobs JJ, Della Valle CJ. Diagnosis and management of adverse local tissue reactions secondary to corrosion at the head-neck junction in patients with metal on polyethylene bearings. J Arthroplasty. 2016;31(1):264-268. doi:10.1016/j.arth.2015.07.039.
- Preuss R, Haeussler KL, Flohr M, Streicher RM. Fretting corrosion and trunnion wear – Is it also a problem for sleeved ceramic heads? Semin Arthroplasty 2012; 23(4): 251-257. doi:10.1053/j.sart.2013.01.008
- Eichler D, Barry J, Lavigne M, Massé V, Vendittoli PA. No radiological and biological sign of trunnionosis with Large Diameter Head Ceramic Bearing Total Hip Arthroplasty after 5 years. Orthop Traumatol Surg Res. 2020;S1877- 0568(20)30045-1. doi:10.1016/j.otsr.2019.12.015
- Koch CN, Figgie M Jr, Figgie MP, Elpers ME, Wright TM, Padgett DE. Ceramic bearings with titanium adapter sleeves implanted during revision hip arthroplasty show minimal fretting or corrosion: a retrieval analysis. HSS J. 2017;13(3):241- 247. doi:10.1007/s11420-017-9566-4.
- MacDonald DW, Chen AF, Lee GC, et al. Fretting and corrosion damage in taper adapter sleeves for ceramic heads: a retrieval study. J Arthroplasty. 2017;32(9):2887-2891. doi:10.1016/j.arth.2017.04.025.
- Kim YH, Park JW, Kim JS. Adapter sleeves are not needed to reduce the risk of fracture of a new ceramic head implanted on a well-fixed stem. Orthopedics. 2018;41(3):158-163. doi:10.3928/01477447-20180501-03.
- Falkenberg A, Dickinson EC, Morlock MM. Adapter sleeves are essential for ceramic heads in hip revision surgery. Clin Biomech (Bristol, Avon). 2020;71:1-4. doi:10.1016/j.clinbiomech.2019.10.018.