In arthroplasty and in dental implantology, implants are identified as foreign bodies by the immune system. A mild immune reaction combined with an appropriate inflammation around the implant serve to protect implants from bacterial attacks for decades. What all orthopedic surgeons and implantologists fear are the pathogenic bacteria, which lead to peri-prosthetic joint infection in orthopedics or in case of oral implants to destructive inflammatory processes known as peri-implant mucositis and peri-implantitis.
Introduction
The clinical success of dental implantology and titanium implants in particular is indisputable. There is evidence of oral implants with a follow-up of 30 years and case reports of survival of over 50 years. This success is intimately linked to the discovery of the osseointegration concept by P.I. Brånemark in 1952 and his following breakthrough research on implants made of biocompatible titanium. Titanium - which is also used in orthopedics - is considered in dental implantology as the gold standard today. Despite these excellent results, implantologists have all been facing cases in which bone and soft tissues surrounding dental implants become inflamed, seemingly infected and in some instances, leading to peri-implant diseases and implant loss. In implantology, we differentiate between peri-implant mucositis and periimplantitis.
Definition
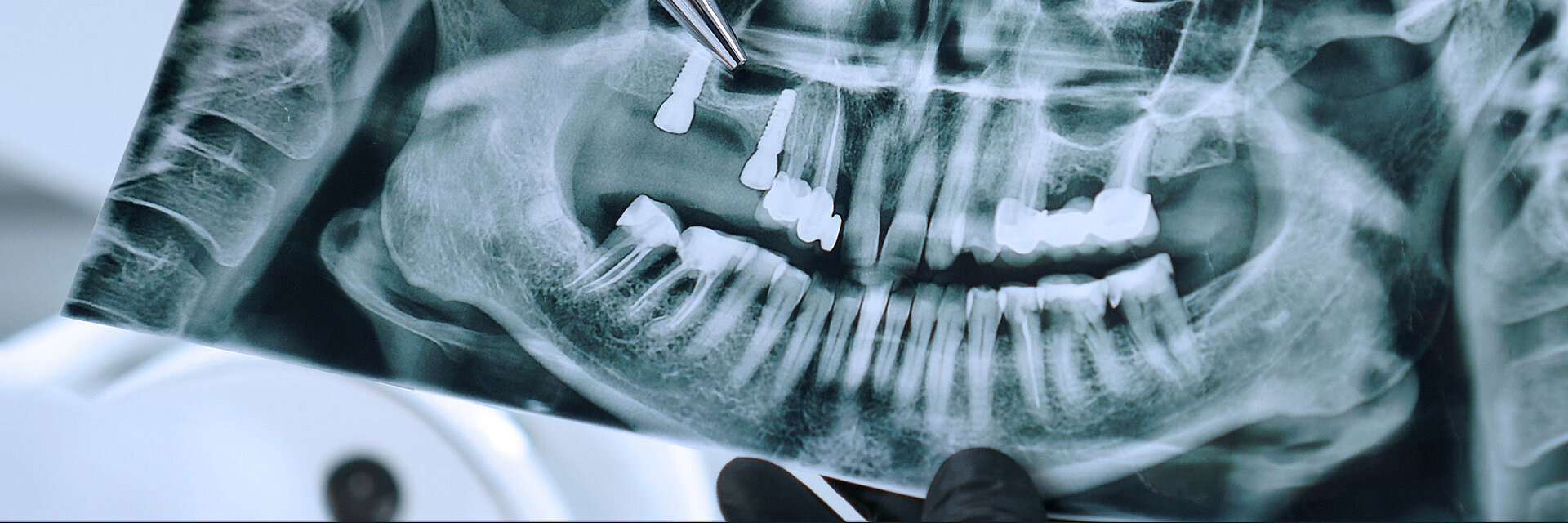
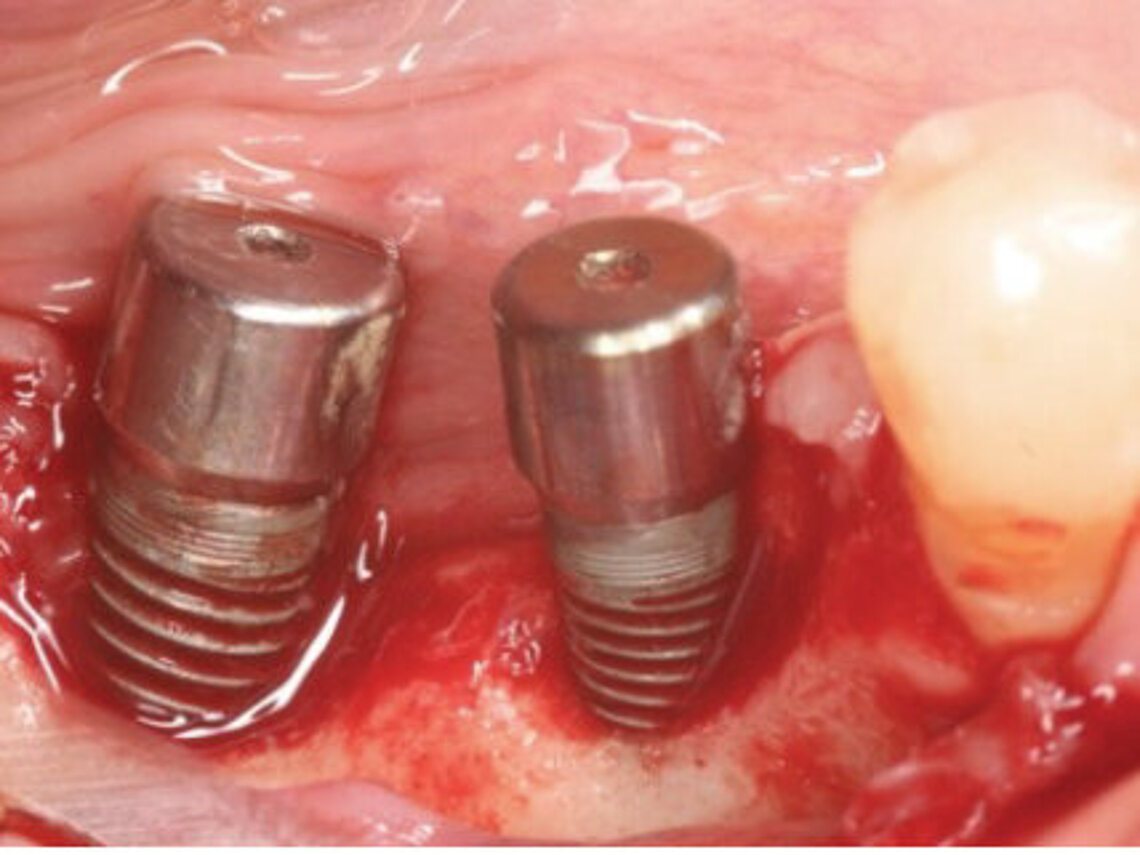
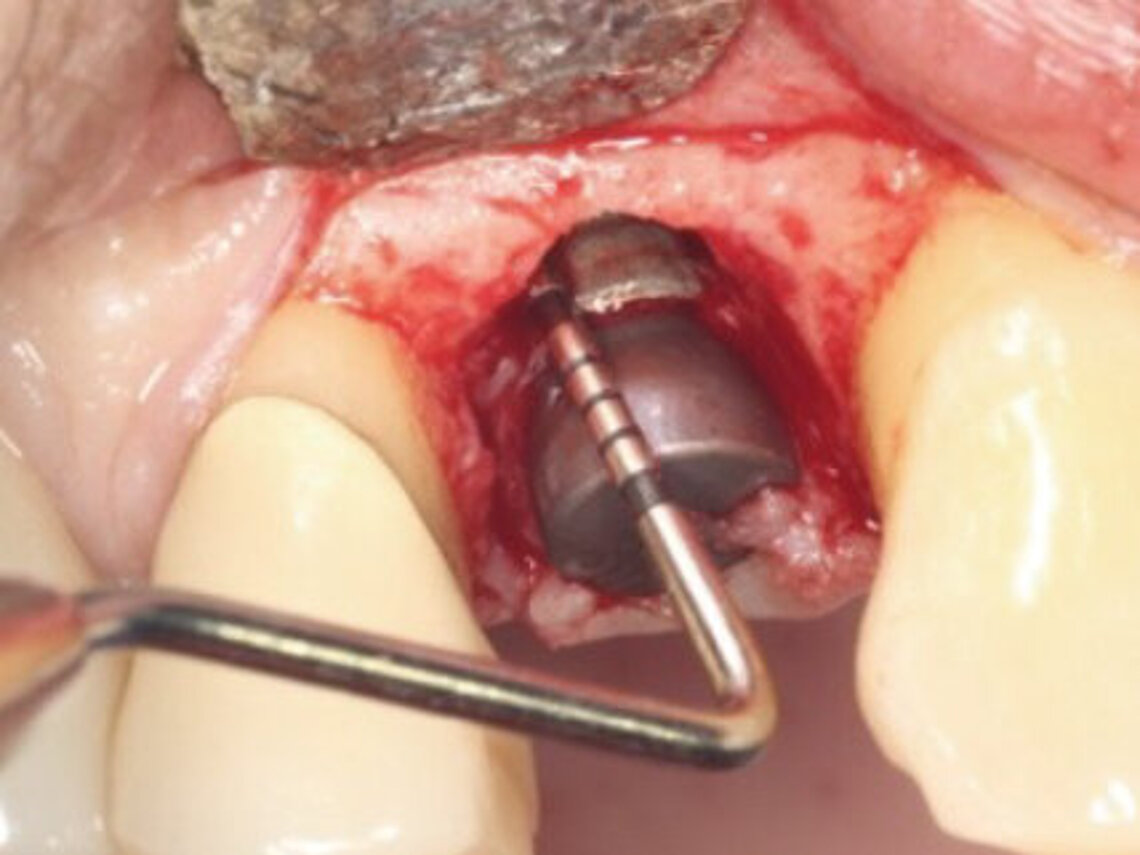
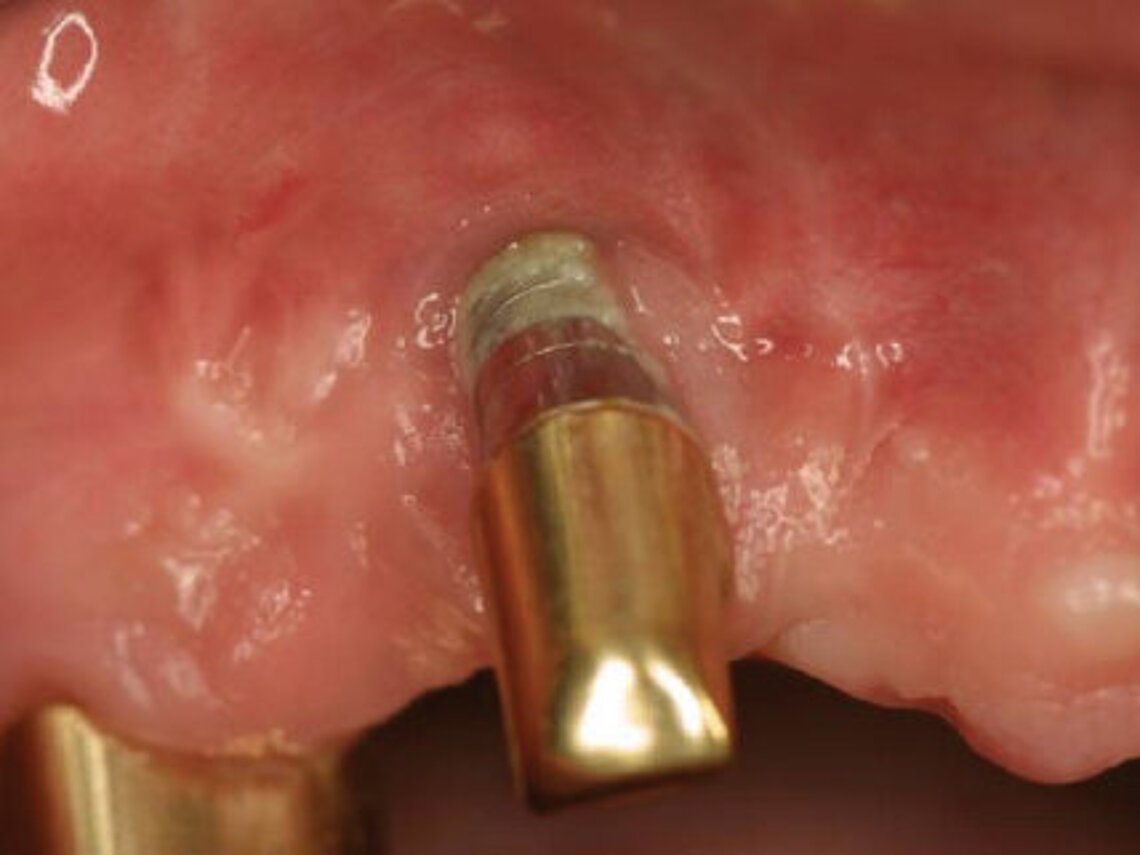
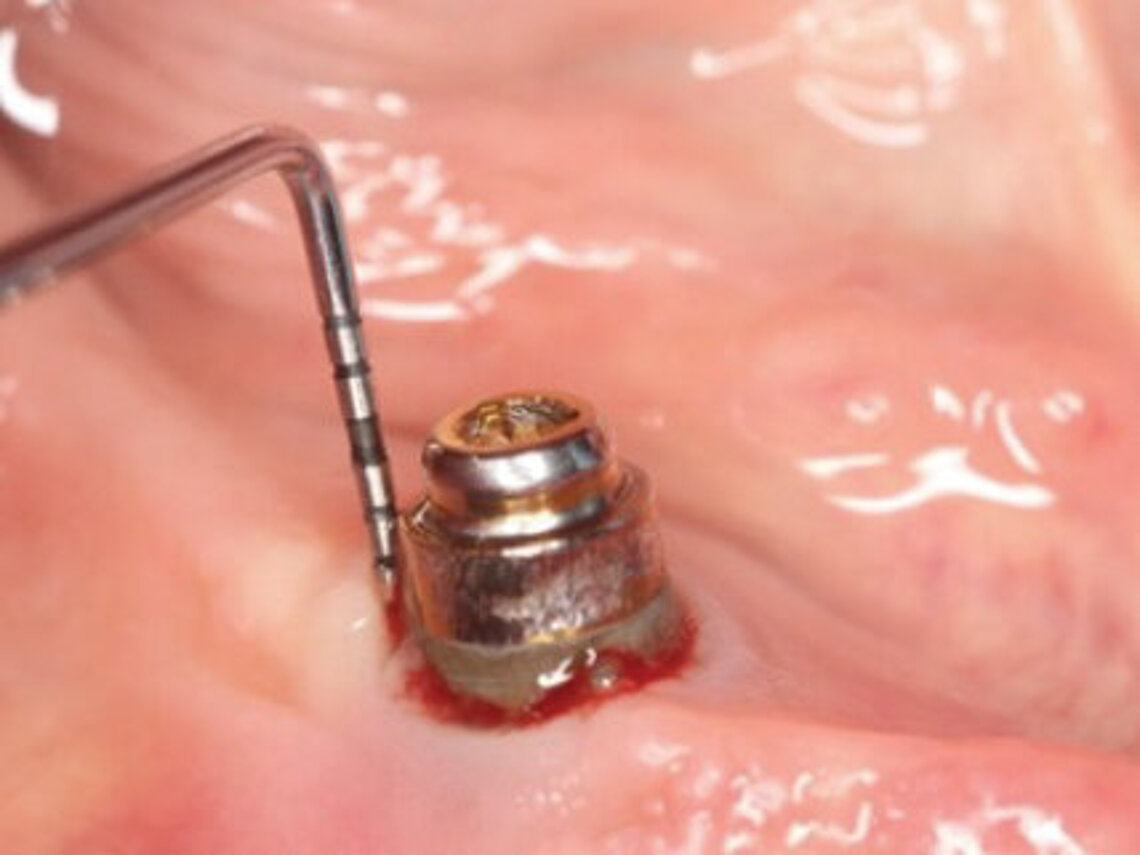
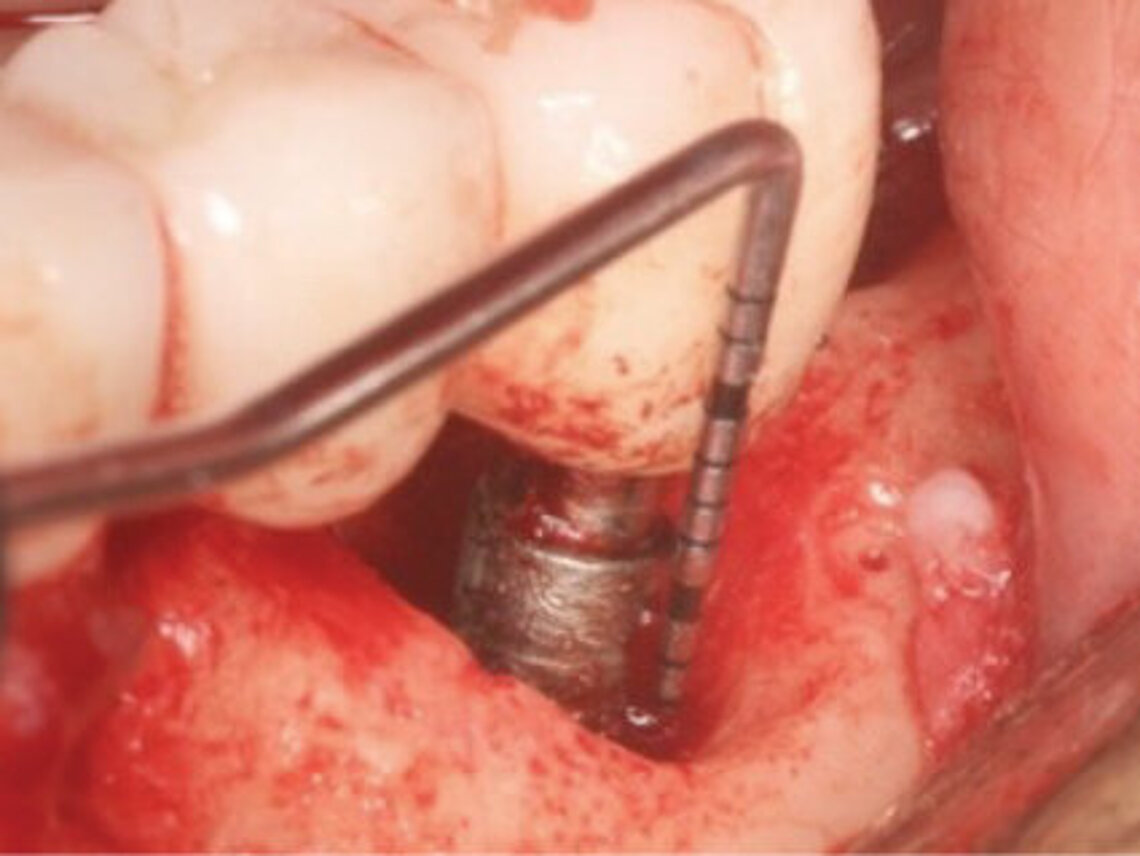
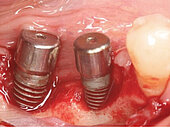
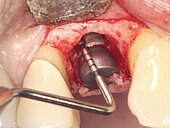
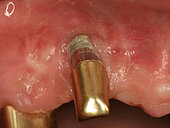
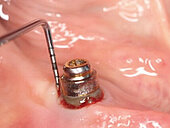
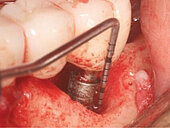
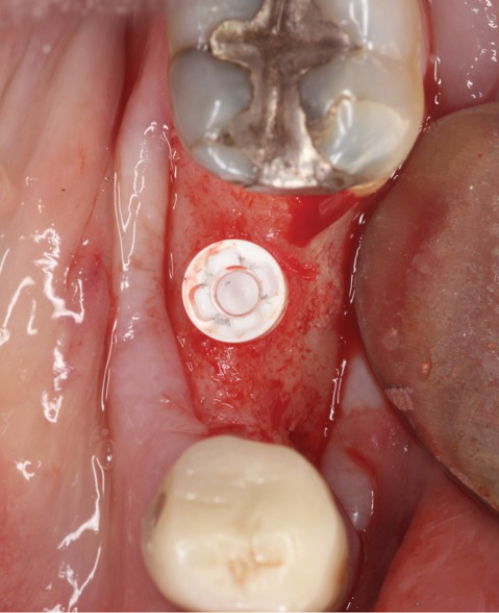
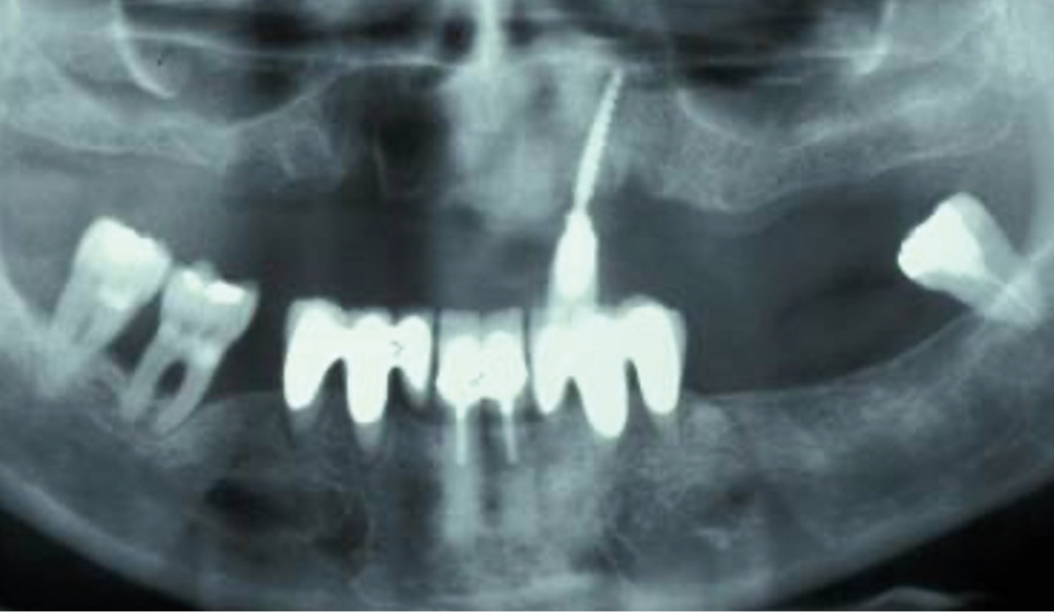
Peri-implant mucositis can be defined as an inflammatory lesion of the soft tissues (mucosa) surrounding the dental implant. Peri-implantitis is an inflammatory lesion of the mucosa affecting the supporting bone (crestal bone) with crestal bone loss and loss of osseointegration. Derks et al. report a prevalence of 43% for peri-implant mucositis and Jepsen et al. estimate that the prevalence of peri-implantitis could reach 22% (Figs. 1, 2).2,3 Peri-implantitis is usually accompanied by crestal bone loss (Fig. 3) and soft tissue changes in the peri-implant sulcus, which can be diagnosed by an increase in bleeding on probing (BOP) more than 5mm over previous examinations and/or suppuration8 (Fig. 4). Also excess of residual cement might contribute to crestal bone loss (Fig. 5).7
The underlying inflammatory processes are still not completely understood. However, increasing evidence shows that the host and the peri-implant conditions might play a pivotal role in the development of peri-implantitis (Figs. 1, 2).2,3
Oral biofilm, oral hygiene, poorly controlled diabetes, smoking and peri-implant plaque have been identified as independent risk factors enabling the development and progression of peri-implantitis. Also, the routinely usage of screw-retained implant-supported restorations seem to be associated with a higher prevalence of peri-implantitis. Having said that, the exact etiology remains often unknown.4-6 And patients with titanium implants and with a good oral hygiene can also develop peri-implantitis.5
Metallosis
When comparing implantology and joint replacement, we can observe similarities in foreign body reaction and failure pattern.9 The release of wear particles and metal ions from a CoCr femoral head in hip replacement can trigger fretting corrosion mechanisms and lead to what surgeons call “taperosis” or “trunnionosis”. In oral implantology, several studies have shown that the release of titanium particles and ions into the surrounding tissue can lead to bone loss around some dental implants.10-14 This process has been named “metallosis”.
Treatment
There are several conservative and surgical approaches available for the treatment of peri-implantitis. Non-surgical therapies are always the preferred treatment, while it is important to maintain implant cleanliness and oral hygiene. However, it is necessary to select approved oral hygiene instruments and to prevent surface damage with consequent generation of titanium particles and release of titanium ions.19-21 Surgical non-regenerative approaches include implant surface decontamination, degranulation of the defect, bone grafting and implantoplasty which can also lead to good clinical outcome.22 There is still ongoing research on methods for the decontamination of dental implants.23 The use of lasers was shown to be potentially beneficial in the treatment of peri-implantitis.24
Ceramic implants as a prevention strategy?
Recently, ceramic implants were introduced as alternative strategy with the aim of preventing the development of peri-implantitis and bone loss (Fig. 6). Compared to titanium implants, studies show that zirconia implants are associated with lower plaque and bleeding scores.15-17 Ceramic implants offer high resistance to corrosion, better peri-implant soft tissue conditions and less inflammation as well as lower oral biofilm adhesion. Apart from the excellent mid-term clinical outcomes, such as a high cumulative survival rate and a low level of average crestal bone loss, zirconia implants contribute to the aesthetics of dental restoration. The white color of zirconia comes close to that of natural teeth.15,18 If the long-term clinical results of ceramic implants are confirmed to be equal to or better than the metal alternative, there is potential for a general switch to ceramics in the future.
Conclusion
Based on current evidence and studies suggesting that zirconia dental implants are associated with less peri-implant inflammatory reactions and less crestal bone loss (Fig. 7), we decided to investigate the behavior, the mechanical stability and the clinical outcomes of ceramic implants at our institution. The first patient implantations look promising. But long-term studies will be required to develop strong evidence and convince the large majority of dental implant surgeons to use zirconia dental implants as routinely as orthopedic surgeons do in hip replacement.
References
1. Brånemark PI, Adell R, Albrektsson T, Lekholm U, Lundkvist S, Rockler B. Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials. 1983;4(1):25-28. doi:10.1016/0142-9612(83)90065-0.
2. Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42 Suppl 16:S158-S171. doi:10.1111/jcpe.12334.
3. Jepsen S, Berglundh T, Genco R, et al. Primary prevention of peri-implantitis: managing peri-implant mucositis. J Clin Periodontol. 2015;42 Suppl 16:S152-S157. doi:10.1111/jcpe.12369.
4. Belibasakis GN, Charalampakis G, Bostanci N, Stadlinger B. Peri-implant infections of oral biofilm etiology. Adv Exp Med Biol. 2015;830:69-84. doi:10.1007/978-3-319-11038-7_4.
5. Javed F, Romanos GE. Evidence-based Implant Dentistry and Systemic Conditions. Wiley Publ., Hoboken, 2018.
6. Máximo MB, de Mendonça AC, Renata Santos V, Figueiredo LC, Feres M, Duarte PM. Short-term clinical and microbiological
evaluations of peri-implant diseases before and after mechanical anti-infective therapies. Clin Oral Implants Res. 2009;20(1):99-108. doi:10.1111/j.1600-0501.2008.01618.x.
7. Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol. 2009;80(9):1388-1392. doi:10.1902/jop.2009.090115.
8. Renvert S, Hirooka H, Polyzois I, Kelekis-Cholakis A, Wang HL; Working Group 3. Diagnosis and non-surgical treatment of peri-implant diseases and maintenance care of patients with dental implants - Consensus report of working group 3. Int Dent J. 2019;69 Suppl 2:12-17. doi:10.1111/idj.12490.
9. Albrektsson T, Becker W, Coli P, Jemt T, Mölne J, Sennerby L. Bone loss around oral and orthopedic implants: An immunologically based condition. Clin Implant Dent Relat Res. 2019;21(4):786-795. doi:10.1111/cid.12793
10. Romanos GE, Delgado-Ruiz R, Sculean A. Concepts for prevention of complications in implant therapy. Periodontol 2000. 2019;81(1):7-17. doi:10.1111/prd.12278.
11. Fretwurst T, Buzanich G, Nahles S, Woelber JP, Riesemeier H, Nelson K. Metal elements in tissue with dental peri-implantitis: a pilot study. Clin Oral Implants Res. 2016;27(9):1178-1186. doi:10.1111/clr.12718.
12. Safioti LM, Kotsakis GA, Pozhitkov AE, Chung WO, Daubert DM. Increased levels of dissolved titanium are associated with peri-Implantitis - A cross-sectional study. J Periodontol. 2017;88(5):436-442. doi:10.1902/jop.2016.160524.
13. Noronha Oliveira M, Schunemann WVH, Mathew MT, et al. Can degradation products released from dental implants affect peri-implant tissues? J Periodontal Res. 2018;53(1):1-11. doi:10.1111/jre.12479.
14. Pettersson M, Pettersson J, Johansson A, Thorén M. Titanium release in peri-implantitis. J Oral Rehabil. 2019; 46: 179–188. Bienz SP, Hilbe M, Hüsler J, Thoma DS, Hämmerle CHF, Jung RE. Clinical and histological comparison of the soft tissue morphology between zirconia and titanium dental implants under healthy and experimental mucositis conditions- A randomized controlled clinical trial. J Clin Periodontol. 2021;48(5):721-733. doi:10.1111/jcpe.13411.
15. Rodriguez AE, Monzavi M, Yokoyama CL, Nowzari H. Zirconia dental implants: A clinical and radiographic evaluation. J Esthet Restor Dent. 2018;30(6):538-544. doi:10.1111/jerd.12414.
16. Bienz SP, Hilbe M, Hüsler J, Thoma DS, Hämmerle CHF, Jung RE. Clinical and histological comparison of the soft tissue morphology between zirconia and titanium dental implants under healthy and experimental mucositis conditions-A randomized controlled clinical trial. J Clin Periodontol. 2021;48(5):721-733. doi:10.1111/jcpe.13411.
17. Sanz-Martín I, Sanz-Sánchez I, Carrillo de Albornoz A, Figuero E, Sanz M. Effects of modified abutment characteristics on peri-implant soft tissue health: A systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(1):118-129. doi:10.1111/clr.13097.
18. Pieralli S, Kohal RJ, Jung RE, Vach K, Spies BC. Clinical outcomes of zirconia dental implants: A systematic review. J Dent Res. 2017;96(1):38-46. doi:10.1177/0022034516664043.
19. Harrel SK, Wilson TG Jr, Pandya M, Diekwisch TGH. Titanium particles generated during ultrasonic scaling of implants. J Periodontol. 2019;90(3):241-246. doi:10.1002/JPER.18-0230.
20. Kotsakis GA, Black R, Kum J, et al. Effect of implant cleaning on titanium particle dissolution and cytocompatibility. J Periodontol. 2021;92(4):580-591. doi:10.1002/JPER.20-0186.
21. Romanos GE, Fischer GA, Delgado-Ruiz R. Titanium wear of dental implants from placement, under loading and maintenance protocols. Int J Mol Sci. 2021;22(3):1067. doi:10.3390/ijms22031067.
22. Keeve PL, Koo KT, Ramanauskaite A, et al. Surgical treatment of periimplantitis with non-augmentative techniques. Implant Dent. 2019;28(2):177-186. doi:10.1097/ID.0000000000000838.
23. Koo KT, Khoury F, Keeve PL, et al. Implant surface decontamination by surgical treatment of periimplantitis: A literature review. Implant Dent. 2019;28(2):173-176. doi:10.1097/ID.0000000000000840.
24. Romanos GE, Gupta B, Yunker M, Romanos EB, Malmstrom H. Lasers use in dental implantology. Implant Dent. 2013;22(3):282-288. doi:10.1097/ID.0b013e3182885fcc.