We live in an era where developments in DNA sequencing and proteomics are allowing researchers to make rapid advances in the understanding of complex disease processes.1 But this technology has not replaced the fundamentals of medical practice. The diagnoses of the most common diseases still rely upon history taking, clinical examination, basic blood tests and imaging. The ability of doctors to understand disease processes, classify them - and thus diagnose them clinically - has to a large extent been developed through the histopathological assessment of diseased tissues. Even in modern medicine, histopathology remains a cornerstone of correctly identifying – and therefore managing - diseases.2
Once a disease process is described and hallmark macro and microscopic features well defined, then further, more specific tests can be developed – either to make it easier for clinicians to diagnose a condition, to identify specific disease features associated with disease progression, or to direct specific treatments. Why is this relevant to the subject of metal allergy following arthroplasty?
Nickel has long been the focus when it comes to discussing metal allergy as a potential cause of arthroplasty failure.3 The diagnosis of nickel allergy has historically been made through the use of skin patch testing4 or lymphocyte transformation tests (LTTs).5 But, to date, there remains no clear understanding of how nickel allergy relates to periprosthetic tissue responses at a microscopic (in terms of well-defined histopathological features) or macroscopic level (the viability of periprosthetic tissues).6, 7 The published evidence supporting the clinical relevance of nickel allergy is at best, even after decades of research, equivocal.4, 6-10 The continued use of the term nickel allergy, and the persisting use of skin patch and LTT tests to identify this ill-defined condition, has led many surgeons to doubt the clinical relevance - if not the very existence - of metal hypersensitivity.11, 12
It could be argued that LTTs and skin patch testing came to be viewed as the gold standard to diagnose metal allergy, before the disease had been well defined (Figure 1) . If we look to specialties outside of orthopaedics, there are several hypersensitivity diseases which have been intensively studied over a number of years.13 These diseases have well defined pathological features, established risk factors, and detailed pathogeneses. In these conditions, it is a well-recognised issue that, in general, skin patch testing and serological studies (including LTTs), identify exposure to an antigen, rather than detecting clinically relevant disease.14 This is further discussed below.
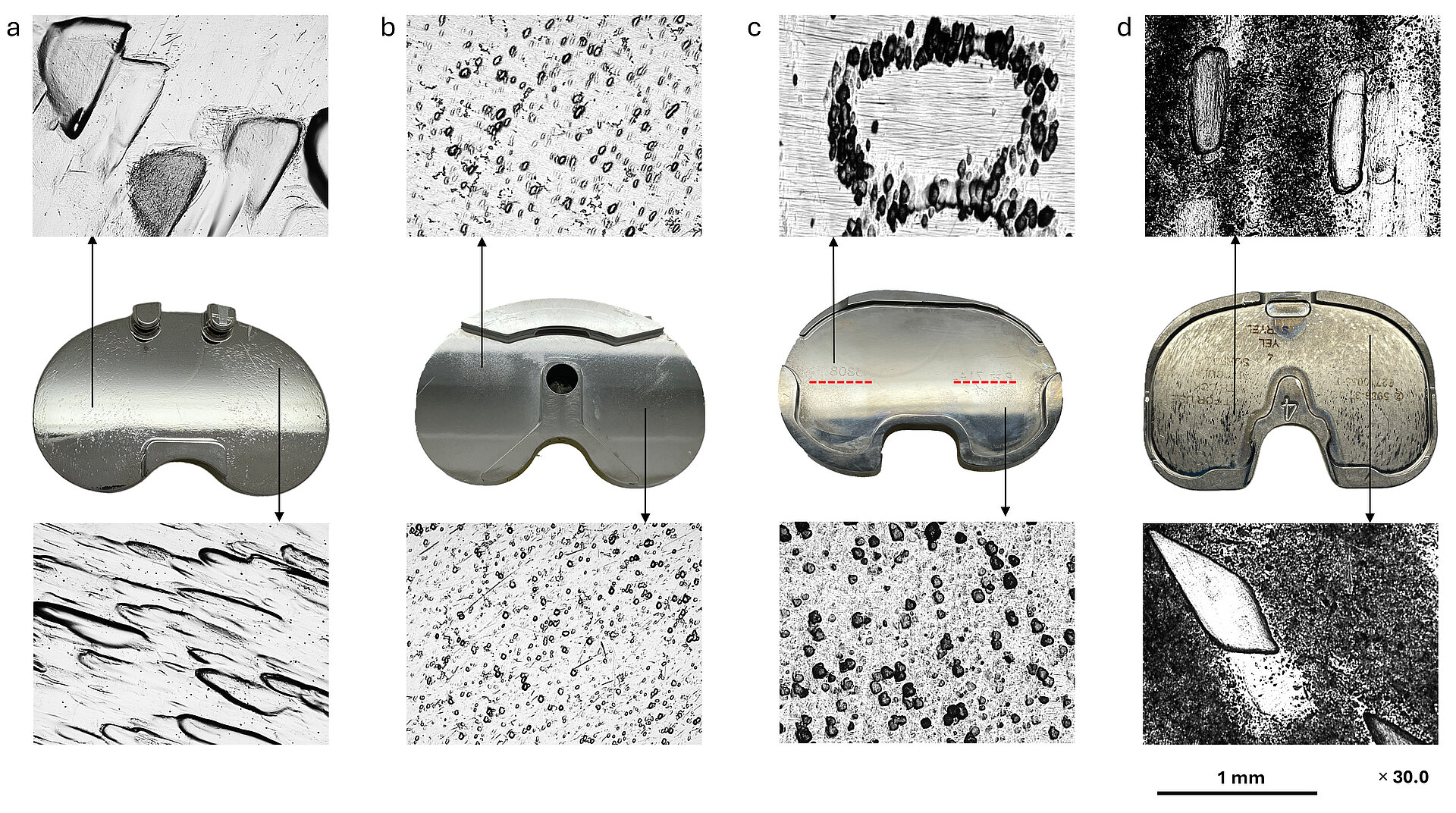
Fig 1: Light microscopic images of CoCr alloy (a and b) and Ti alloy (c and d) tibial trays showing pitting on the superior surface. Red dotted lines on Ti alloy tray c indicate imprinting of the lot number marking of the polyethylene insert on the superior surface of the tray. Pitting is present on over 50% of explanted trays we receive at ExplantLab.
The failure of modern metal-on-metal hip arthroplasty was not a high point in the history of orthopaedic surgery.15 But one of the few positives that can be taken from the experience is the knowledge the orthopaedic community developed with regard to the understanding of immune responses to implants. Circa 2005, Willert and Davies provided a detailed description of the pathological responses seen in association with failed metal-on-metal hip prostheses.16 These pathological features included prominent perivascular lymphocyte infiltration, formation of high endothelial venules, accumulation of sheets of macrophages and surface membrane necrosis.
The acronym ALVAL (“aseptic lymphocyte dominated vasculitis associated lesion”) was coined. Clearly, ALVAL was a newly recognised condition.
As Davies wrote:
Following Willert et al’s publications, clinicians and researchers now had a defined disease, with defined pathological features that could be quantified on tissue specimens. Because of this, and in addition to the large number of patients who presented to clinics around the world experiencing complications from metal-related pathology, a substantial amount of knowledge was gained over a short period of time in the understanding of ALVAL. It is important to note, however, that in their seminal 2008 paper on pseudotumour development following metal-on-metal hip resurfacing, the Oxford group speculated that these (at times, catastrophic) periprosthetic reactions could be due to nickel allergy. To reiterate the point: metal-on-metal failure was originally proposed to be due to nickel allergy.18 It is also crucial to note that when the first reports of metal-on-metal hip failures began to emerge, blood metal ion testing was not routinely performed nor believed to be of benefit in patient management.
At around the same period in time, in our hospital we began to see an ever increasing number of patients who developed ALVAL responses to their ASR hips.19 In an attempt to find answers, we established a retrieval laboratory to measure the volumetric material loss from the bearing and taper surfaces of explanted devices.20 These values were compared to synovial, blood and serum metal ion concentrations and, most importantly, to the associated periprosthetic tissue responses. From the results, it became clear that our patients were developing aggressive ALVAL responses to abnormally functioning prostheses.21
The issue of nickel allergy was still at the forefront of our thoughts, however. We carried out a small study to investigate the possibility that LTT testing may show that these patients were sensitive to nickel.21 We performed LTTs on blood samples obtained from a group of patients who had experienced failure of their ASRs secondary to ALVAL. We then compared these results to a control group who had elevated blood metal ion concentrations but were symptom free, with no abnormalities detected on cross sectional imaging. In the ALVAL group, only one of six patients tested positive for metal sensitivity on the LTT tests, whereas three of the five patients in the control group tested positive for sensitivity to a range of metals (all positive for nickel). The Oxford group, who had led the world in reporting their investigation of pseudotumours, carried out a similar study with larger numbers.22 Their findings were consistent with ours.
Since Davies and Willert published their work16, 17, there have been over a thousand papers published on ALVAL, submitted from hospital and research centres all over the world. This global effort has allowed the following facts to be established:
- ALVAL is a well-defined condition, in a histopathological sense.16 The pathological features are consistent with a delayed type hypersensitivity response and, as would be expected with delayed type hypersensitivity, reimplantation with cobalt-chrome alloy is ill advised, with symptoms returning after temporary alleviation.16, 19
- ALVAL varies in intensity; multiple grading systems exist.23-25 These grading systems largely agree with each other26, 27, as they are generally in unison on scoring the same parameters; the primary one being the presence of lymphocyte perivascular cuffing.16
- ALVAL has most frequently been identified in studies involving patients with metal-on-metal prostheses. However, a metal-on-metal bearing is not a prerequisite for the condition to develop28-30, and ALVAL has been frequently identified in tissues excised during knee revision surgery.31
- ALVAL is associated with abnormal fluid collections (which can build in pressure), and soft tissue and/or bone damage which can be extensive.30
- The grade of ALVAL correlates with the thickness of synovial lining on MRI32, the extent of surface membrane necrosis of the synovium33 and the level of pain reported by the patient prior to revision.34, 35
- ALVAL is associated with elevations in blood, serum and synovial cobalt and chromium concentrations.23, 25, 36
Regarding the final point, for over a decade we continued to collect data to study the interaction between patient and implant factors and the development of ALVAL in hundreds of patients. We found that while ALVAL was more common in devices generating excessive metal debris, beyond that, there appeared to be immunogenetic factors at play, with different patients displaying different tolerances before developing hypersensitivity.37
There are a host of hypersensitivity diseases which can afflict the human body. They tend to share certain features, however. As Friedmann made clear: “age, sex and genetic factors such as HLA haplotype can all influence whether and to what degree an individual reacts to a substance.”38
As just one example (there are many13), bird fancier’s lung is a common form of delayed type hypersensitivity pneumonitis that results from avian antigen exposure. It is related to the dose and duration of exposure39, 40, age and sex41, but also depends on an individual’s HLA genes.40, 42
Given the existing knowledge regarding hypersensitivity diseases, in 2017, after a decade of data collection, we set about identifying patients with extreme phenotypes in an attempt to identify HLA genetic variants associated with ALVAL. Extreme phenotypes in this respect referred to those patients developing high grade ALVAL reactions following relatively low metal exposure, versus those who appeared resistant to developing ALVAL despite long term exposure to large amounts of metal debris.
We quickly identified significant differences in the frequencies of specific HLA-DQ alleles, even with relatively small patient numbers. Not only that, but the peptide binding groove encoded by the primary HLA-DQ risk haplotype demonstrated an affinity for the N-terminal peptide fragment sequence of albumin, a recognised metal ion binding site.43 These binding affinities, along with patient (age and sex) and implant factors (duration in vivo, presence of modular junction) were entered into a machine learning algorithm which was trained and tested using the largest dataset of patients in the published literature.44 Extension of the genetic testing to include the whole genome did not identify genetic variants that improved the algorithm to any clinically relevant degree. The resulting commercial test "Orthotype” is now available for surgeons in the United Kingdom to use. The evidence validating Orthotype has been reviewed by the UK National Health Service and the test is provided by the NHS Blood and Transplant service. (Find out more)
Orthotype is a test which can be used pre- or post-operatively. It involves a routine blood draw, with samples placed in EDTA tubes and transported at ambient temperature.
With Orthotype Pre-Op, the patient’s HLA-DQ alleles are identified using next-generation sequencing. The resulting haplotypes are then inputted into a machine learning algorithm, along with patient age, sex and device type, to provide the clinician with a relative risk over time (compared to the background population) of the patient developing ALVAL in response to a cobalt-chrome implant.
Orthotype Post-Op can be used to monitor or investigate patients who have already received a cobalt-chrome implant. With this test, blood metal ion levels concentrations are measured using the same samples, and these results are added into the algorithm to provide the clinician with a result: ARMD positive or negative. If positive, a graph is outputted which allows the clinician to view the risk of ALVAL developing over time. The sensitivity and specificity for detection of ARMD post-op is 89% (84-93) and 90% (87-92), with a weighted mean survival probability error of 1.8% for pre-op prediction of ALVAL at 1.8% and 3.1% for post-op ALVAL.44
Orthotype was developed and validated using gold standard histopathological examination of tissue excised at revision surgery. The validation process was performed stepwise, with the candidate genetic variants identified first, then an algorithm developed and tested using prospectively collected data from three different countries. Histology results from 176 revision surgeries were included in the data set, along with clinical results from 430 patients followed up to a maximum of twenty years.44 The same methodology was used in the field of infectious disease to successfully identify genetic variants which confer protection from severe complications caused by COVID.45, 46
The originally published validation study included only patients with metal-on-metal THRs or resurfacings. However, a national study is currently being carried out to provide additional prospective data with patients implanted with other joint prostheses. The test is performing in line with the originally stated sensitivity and specificity values.
As discussed above, the phenomenon of “nickel allergy” has become inextricably linked with skin patch testing and LTTs. But the limitations of these tests as diagnostic tools are well recognised in other fields of medicine. For example, chronic beryllium disease is a pulmonary hypersensitivity disease resulting from exposure to the heavy metal, beryllium. In this HLA mediated condition, exposure to beryllium leads to a cell-mediated immune response where T-cells become sensitised to beryllium resulting in progressive lung damage.47 LTT screening of exposed workers commonly identifies individuals sensitised to the metal who have no evidence of lung disease. In other words, these “sensitised” subjects have a LTT beryllium specific response in peripheral blood, but no clinical or pathologic features of lung pathology.48 A positive LTT in beryllium disease therefore indicates sensitisation, rather than the presence of pulmonary disease.49 This effect is seen in other pulmonary hypersensitivity conditions, with positive skin patch and serological tests in up to 88% of exposed subjects who remain asymptomatic without evidence of disease.14
What is the implication of this? In orthopaedics, we may have used measures of exposure or sensitisation to diagnose disease, rather than identifying disease and then developing diagnostic tests to identify disease. If this is indeed the case, it would explain why these tests have not been proven to accurately identify ALVAL or tissue related complications necessitating revision. The damaging knock-on effect has been to create scepticism that metal hypersensitivity even exists. Rather than doubting the accuracy or appropriateness of the tests, the doubt has been cast on the disease.11
How can we unify the research findings and improve the terminology? If we restrict ourselves to using evidence derived from a classical approach to medicine (signs, symptoms, biochemistry, imaging and histopathology), then it is clear that the evidence indicates that it is ALVAL that is the condition which constitutes a clinically relevant meaningful disease process; the condition which we should be aiming to identify and eliminate moving forward.
ALVAL is present in approximately 30% of tissue samples taken from patients undergoing revision of their knee arthroplasties29, 31, 35 and the grade of ALVAL correlates to the levels of pain reported by patients prior to their revision procedures.34, 35 There is even evidence that ALVAL may masquerade as culture negative prosthetic joint infections, just as was the case with the first metal-on-metal ALVAL reactions encountered by unsuspecting surgeons.31, 50 So why is this condition still viewed with scepticism? Why is ALVAL apparently siloed in the world of failing metal-on-metal hips, and what are the obstacles preventing it gaining attention in other areas of arthroplasty?
The issue of diagnosis
ALVAL is diagnosed through histopathology. Being a relatively novel condition, many non-specialist units may not be aware that ALVAL exists nor have the relevant expertise to identify it. In the UK, most knee surgeons do not send a periprosthetic tissue sample at revision for histological examination. The incidence and clinical significance of ALVAL therefore by definition cannot be determined (in the UK at least) within the bounds of current clinical practice.
A lack of awareness of evidence and persisting, inaccurate beliefs
There is no established culture in current knee arthroplasty practice to measure blood metal ion concentrations post-operatively, and metal hypersensitivity is regarded as a diagnosis of exclusion.51 In these respects, there are striking parallels with the early management of patients with metal-on-metal hips. There remains a fixed belief that metal debris generation from a contemporary knee arthroplasty is clinically insignificant. However, a cursory examination of the literature on the subject identifies evidence to challenge these assumptions. Blood metal ions are frequently elevated to clinically relevant concentrations post knee arthroplasty.52-55 Sceptics may suggest that these elevations only occur with defective or failing devices. Yet evidence shows that even in the idealised environment of a knee simulator, approximately 12 % by weight of the wear products generated from a knee arthroplasty is metallic.56 Furthermore, concerns have been expressed that the increased wear resistance of contemporary polyethylene changes may alter force transmission through the links of the arthroplasty chain, resulting in a change in the magnitude of wear debris generated at each interface.57
The unexplained phenomenon of nickel allergy in knees versus cobalt-chrome associated ALVAL in hips
It is now clear that excessively wearing cobalt-chrome hip components precipitate high rates of ALVAL.37, 58The standard cobalt-chrome alloy used in metal-on-metal hips (ASTM 75) is identical to that used in the vast majority of knee prostheses.59 These cobalt-chrome alloys rarely contain more than 0.2% nickel by weight, compared to up to 65% of cobalt - amounting to a three hundred fold difference. To place nickel under scrutiny seems questionable. As another comparison, an Exeter hip contains fifty times the amount of nickel compared to a cobalt-chrome component. Global clinical experience has demonstrated which devices are more likely to provoke an ALVAL response. That is not to say that nickel associated ALVAL doesn’t exist - far from it – but, just as with other diseases, histopathological analysis should form a cornerstone of the diagnostic process.2
To summarise, the available evidence indicates that ALVAL is the histopathological manifestation of delayed type metal hypersensitivity. Evidence also indicates that debris produced from cobalt-chrome alloy is the most relevant sensitising agent from a clinical perspective. Could it be cobalt, chromium, nickel or perhaps one of the other elemental constituents of cobalt-chrome alloy (such as molybdenum) that is the major culprit? Does it matter? The most relevant clinical question seems to centre on other orthopaedic biomaterials, most notably titanium alloy. At our unit, and as part of the ongoing national Arthrogenex study, we are actively investigating titanium associated ALVAL. It certainly exists.
Reassuringly, it appears that the genetic risk factors associated with ALVAL secondary to cobalt- chrome exposure are not the same as those associated with titanium-induced ALVAL. Does this mean that future surgical practice may be based on biomaterial selection guided by an individual’s DNA profile? Why not? This is already happening in other specialties to guide pharmacological treatment regimes.60-63
References
1. Roehrl MH, Roehrl VB, Wang JY. Proteome-based pathology: the next frontier in precision medicine. Expert review of precision medicine and drug development. 2021;6(1):1-4.
2. Underwood JC. More than meets the eye: the changing face of histopathology. Histopathology. 2017;70(1):4-9.
3. Ring PA. Replacement of the hip joint. Annals of the Royal College of Surgeons of England. 1971;48(6):344-55.
4. Bogdanova-Bennett A, Sagi A, Asopa V, Field RE, Sochart DH. Nickel hypersensitivity and skin patch testing in total hip replacement surgery: a systematic review. EFORT open reviews. 2021;6(10):825-38.
5. Schneiderman BA, Yang S, Dipane M, Lu C, McPherson EJ, Schmalzried TP. Periprosthetic Tissue Reaction Independent of LTT Result and Implanted Materials in Total Knee Arthroplasty. J Arthroplasty. 2021;36(7):2480-5.
6. Bravo D, Wagner ER, Larson DR, Davis MP, Pagnano MW, Sierra RJ. No Increased Risk of Knee Arthroplasty Failure in Patients With Positive Skin Patch Testing for Metal Hypersensitivity: A Matched Cohort Study. J Arthroplasty. 2016;31(8):1717-21.
7. Peacock CJH, Fu H, Asopa V, Clement ND, Kader D, Sochart DH. The effect of Nickel hypersensitivity on the outcome of total knee arthroplasty and the value of skin patch testing: a systematic review. Arthroplasty (London, England). 2022;4(1):40.
8. Bracey DN, Hegde V, Johnson R, Kleeman-Forsthuber L, Jennings J, Dennis D. Poor Correlation Among Metal Hypersensitivity Testing Modalities and Inferior Patient-Reported Outcomes After Primary and Revision Total Knee Arthroplasties. Arthroplasty today. 2022;18:138-42.
9. Abouharb A, Joseph PJS, Pandit H. Existing and Novel Assessment Methods for Metal Sensitivity in Elective Lower-Limb Arthroplasty-A Scoping Review. Arthroplasty today. 2024;28:101462.
10. Lionberger DR, Samorajski J, Wilson CD, Rivera A. What role does metal allergy sensitization play in total knee arthroplasty revision? Journal of experimental orthopaedics. 2018;5(1):30.
11. Innocenti M, Vieri B, Melani T, Paoli T, Carulli C. Metal hypersensitivity after knee arthroplasty: fact or fiction? Acta bio-medica : Atenei Parmensis. 2017;88(2S):78-83.
12. Hallock K, Vaughn NH, Juliano P, Marks JG, Jr. Metal Hypersensitivity and Orthopedic Implants: Survey of Orthopedic Surgeons. Dermatitis. 2017;28(1):76-80.
13. Riario Sforza GG, Marinou A. Hypersensitivity pneumonitis: a complex lung disease. Clinical and Molecular Allergy. 2017;15(1):6.
14. Chan AL, Juarez MM, Leslie KO, Ismail HA, Albertson TE. Bird fancier's lung: a state-of-the-art review. Clinical reviews in allergy & immunology. 2012;43(1-2):69-83.
15. Cohen D. How safe are metal-on-metal hip implants? Bmj. 2012;344:e1410.
16. Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. The Journal of bone and joint surgery American volume. 2005;87.
17. Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An Unusual Lymphocytic Perivascular Infiltration in Tissues Around Contemporary Metal-on-Metal Joint Replacements. JBJS. 2005;87(1):18-27.
18. Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, et al. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90(7):847-51.
19. Jameson S, Langton D, Nargol A. Articular surface replacement of the hip: a prospective single-surgeon series. The Journal of bone and joint surgery British volume. 2010;92(1):28-37.
20. Langton D, Sidaginamale R, Holland J, Deehan D, Joyce T, Nargol A, et al. Practical considerations for volumetric wear analysis of explanted hip arthroplasties. Bone & joint research. 2014;3(3):60-8.
21. Langton D, Jameson S, Joyce T, Hallab N, Natu S, Nargol A. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. The Journal of bone and joint surgery British volume. 2010;92(1):38-46.
22. Kwon YM, Thomas P, Summer B, Pandit H, Taylor A, Beard D, et al. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;28(4):444-50.
23. Langton D, Sidaginamale R, Joyce T, Bowsher J, Holland J, Deehan D, et al. Aseptic lymphocyte-dominated vasculitis-associated lesions are related to changes in metal ion handling in the joint capsules of metal-on-metal hip arthroplasties. Bone & joint research. 2018;7(6):388-96.
24. Campbell P, Ebramzadeh E, Nelson S, Takamura K, Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clinical orthopaedics and related research. 2010;468.
25. Grammatopoulos G, Pandit H, Kamali A, Maggiani F, Glyn-Jones S, Gill HS. The correlation of wear with histological features after failed hip resurfacing arthroplasty. The Journal of bone and joint surgery American volume. 2013;95.
26. Phillips EA, Klein GR, Cates HE, Kurtz SM, Steinbeck M. Histological characterization of periprosthetic tissue responses for metal-on-metal hip replacement. Journal of long-term effects of medical implants. 2014;24(1):13-23.
27. Bauer TW, Zhang Y, Gao MA, Lin BQ, Koff MF. Reproducibility of pathologic scoring systems for periprosthetic adverse local tissue reactions: A cross-sectional study. Pathology - Research and Practice. 2021;228:153685.
28. Fujishiro T, Moojen DJ, Kobayashi N, Dhert WJ, Bauer TW. Perivascular and diffuse lymphocytic inflammation are not specific for failed metal-on-metal hip implants. Clinical orthopaedics and related research. 2011;469(4):1127-33.
29. Ng VY, Lombardi AV, Jr., Berend KR, Skeels MD, Adams JB. Perivascular lymphocytic infiltration is not limited to metal-on-metal bearings. Clinical orthopaedics and related research. 2011;469(2):523-9.
30. Burge AJ, Gold SL, Lurie B, Nawabi DH, Fields KG, Koff MF, et al. MR Imaging of Adverse Local Tissue Reactions around Rejuvenate Modular Dual-Taper Stems. Radiology. 2015;277(1):142-50.
31. Rajamäki A, Lehtovirta L, Niemeläinen M, Reito A, Parkkinen J, Peräniemi S, et al. Mild aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL)-type reactions also present in patients with failed knee prostheses. Bone Joint Res. 2024;13(4):149-56.
32. Koff MF, Esposito C, Shah P, Miranda M, Baral E, Fields K, et al. MRI of THA Correlates With Implant Wear and Tissue Reactions: A Cross-sectional Study. Clinical orthopaedics and related research. 2019;477(1):159-74.
33. Natu S, Sidaginamale RP, Gandhi J, Langton DJ, Nargol AV. Adverse reactions to metal debris: histopathological features of periprosthetic soft tissue reactions seen in association with failed metal on metal hip arthroplasties. Journal of clinical pathology. 2012;65(5):409-18.
34. Kurmis AP, Herman A, McIntyre AR, Masri BA, Garbuz DS. Pseudotumors and High-Grade Aseptic Lymphocyte-Dominated Vasculitis-Associated Lesions Around Total Knee Replacements Identified at Aseptic Revision Surgery: Findings of a Large-Scale Histologic Review. J Arthroplasty. 2019;34(10):2434-8.
35. Crawford DA, Passias BJ, Adams JB, Berend KR, Lombardi AV. Impact of perivascular lymphocytic infiltration in aseptic total knee revision. The bone & joint journal. 2021;103-b(6 Supple A):145-9.
36. Sheridan GA, Neufeld ME, Sidhu A, Kurmis AP, Kelly M, O'Byrne JM, et al. The Diagnostic Utility of Serum Metal Ion Markers for High-Grade Aseptic Lymphocyte-Dominated Vasculitis-Associated Lesions (ALVALs) in Revision Hip and Knee Arthroplasty: An International Multicenter Study. J Arthroplasty. 2024;39(1):206-10.
37. Langton D, Joyce T, Jameson S, Lord J, Van Orsouw M, Holland J, et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear (vol 93, pg 164, 2011). JOURNAL OF BONE AND JOINT SURGERY-BRITISH VOLUME. 2011;93(4):566-.
38. Friedmann PS. The relationships between exposure dose and response in induction and elicitation of contact hypersensitivity in humans. British Journal of Dermatology. 2007;157(6):1093-102.
39. Cramer C, Schlünssen V, Bendstrup E, Stokholm ZA, Vestergaard JM, Frydenberg M, et al. Risk of hypersensitivity pneumonitis and interstitial lung diseases among pigeon breeders. The European respiratory journal. 2016;48(3):818-25.
40. Judson MA, Sahn SA. Bird-years as well as pack-years. Chest. 2004;125(1):353-4.
41. Kawano-Dourado L, Glassberg MK, Assayag D, Borie R, Johannson KA. Sex and gender in interstitial lung diseases. European respiratory review : an official journal of the European Respiratory Society. 2021;30(162).
42. Churg A. Hypersensitivity pneumonitis: new concepts and classifications. Modern Pathology. 2022;35:15-27.
43. Sokołowska M, Wszelaka-Rylik M, Poznański J, Bal W. Spectroscopic and thermodynamic determination of three distinct binding sites for Co(II) ions in human serum albumin. Journal of inorganic biochemistry. 2009;103(7):1005-13.
44. Langton DJ, Bhalekar RM, Joyce TJ, Rushton SP, Wainwright BJ, Nargol ME, et al. The influence of HLA genotype on the development of metal hypersensitivity following joint replacement. Communications medicine. 2022;2:73.
45. Langton DJ, Bourke SC, Lie BA, Reiff G, Natu S, Darlay R, et al. The influence of HLA genotype on the severity of COVID-19 infection. Hla. 2021;98(1):14-22.
46. Augusto DG, Murdolo LD, Chatzileontiadou DSM, Sabatino JJ, Jr., Yusufali T, Peyser ND, et al. A common allele of HLA is associated with asymptomatic SARS-CoV-2 infection. Nature. 2023;620(7972):128-36.
47. Fontenot AP, Falta MT, Kappler JW, Dai S, McKee AS. Beryllium-Induced Hypersensitivity: Genetic Susceptibility and Neoantigen Generation. Journal of immunology (Baltimore, Md : 1950). 2016;196(1):22-7.
48. Newman LS, Mroz MM, Balkissoon R, Maier LA. Beryllium sensitization progresses to chronic beryllium disease: a longitudinal study of disease risk. American journal of respiratory and critical care medicine. 2005;171(1):54-60.
49. Balmes JR, Abraham JL, Dweik RA, Fireman E, Fontenot AP, Maier LA, et al. An official American Thoracic Society statement: diagnosis and management of beryllium sensitivity and chronic beryllium disease. American journal of respiratory and critical care medicine. 2014;190(10):e34-59.
50. Jameson S, Langton D, Nargol A, editors. ASR HIP RESURFACING: AN INDEPENDENT, SINGLE-SURGEON PROSPECTIVE STUDY OF THE FIRST 214 HIPS. Orthopaedic Proceedings; 2010: The British Editorial Society of Bone & Joint Surgery.
51. van der Merwe JM. Metal Hypersensitivity in Joint Arthroplasty. Journal of the American Academy of Orthopaedic Surgeons Global research & reviews. 2021;5(3).
52. Savarino L, Tigani D, Greco M, Baldini N, Giunti A. The potential role of metal ion release as a marker of loosening in patients with total knee replacement: a cohort study. J Bone Joint Surg Br. 2010;92(5):634-8.
53. Luetzner J, Krummenauer F, Lengel AM, Ziegler J, Witzleb WC. Serum metal ion exposure after total knee arthroplasty. Clinical orthopaedics and related research. 2007;461:136-42.
54. Lons A, Putman S, Pasquier G, Migaud H, Drumez E, Girard J. Metallic ion release after knee prosthesis implantation: a prospective study. International orthopaedics. 2017;41(12):2503-8.
55. Lukas S, Martinot P, Putman S, Lons A, Drumez E, Migaud H, et al. Metal ion release after hip resurfacing arthroplasty and knee arthroplasty: a retrospective study of one hundred ninety-five cases. International orthopaedics. 2024;48(1):119-26.
56. Kretzer JP, Reinders J, Sonntag R, Hagmann S, Streit M, Jeager S, et al. Wear in total knee arthroplasty--just a question of polyethylene?: Metal ion release in total knee arthroplasty. International orthopaedics. 2014;38(2):335-40.
57. Bhalekar RM, Wells SR, Matthew Nargol M, Shariatpanahi S, Nargol AVF, Waller S, et al. Aseptic loosening of the option stemmed tibial tray in the Zimmer NexGen LPS total knee arthroplasty system. The Knee. 2024;47:1-12.
58. Nodzo SR, Esposito CI, Potter HG, Ranawat CS, Wright TM, Padgett DE. MRI, Retrieval Analysis, and Histologic Evaluation of Adverse Local Tissue Reaction in Metal-on-Polyethylene Total Hip Arthroplasty. J Arthroplasty. 2017;32(5):1647-53.
59. Bhalekar RM, Nargol ME, Shyam N, Nargol AVF, Wells SR, Collier R, et al. Tibial tray debonding from the cement mantle is associated with deformation of the backside of polyethylene tibial inserts. The bone & joint journal. 2021;103-B(12):1791-801.
60. Pereira NL, Cresci S, Angiolillo DJ, Batchelor W, Capers Qt, Cavallari LH, et al. CYP2C19 Genetic Testing for Oral P2Y12 Inhibitor Therapy: A Scientific Statement From the American Heart Association. Circulation. 2024;150(6):e129-e50.
61. Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Archives of pathology & laboratory medicine. 2018;142(3):321-46.
62. Kurnit KC, Fleming GF, Lengyel E. Updates and New Options in Advanced Epithelial Ovarian Cancer Treatment. Obstetrics and gynecology. 2021;137(1):108-21.
63. Hewitt DB, Aziz H, Brown ZJ, Pawlik TM. Role of genetic testing in hepatic, pancreatic, and biliary cancers. Surgical oncology. 2022;44:101844.


