Demonstrating Clinical Excellence: 50 Years of BIOLOX® Performance
For half a century, BIOLOX® has been at the forefront of developing and manufacturing medical bioceramics, delivering products whose performance is backed by extensive clinical evidence. Our commitment to excellence is reflected in the delivery of over 28 million components worldwide, a testament to the trust that clinicians and patients place in BIOLOX®.
Trusted Worldwide
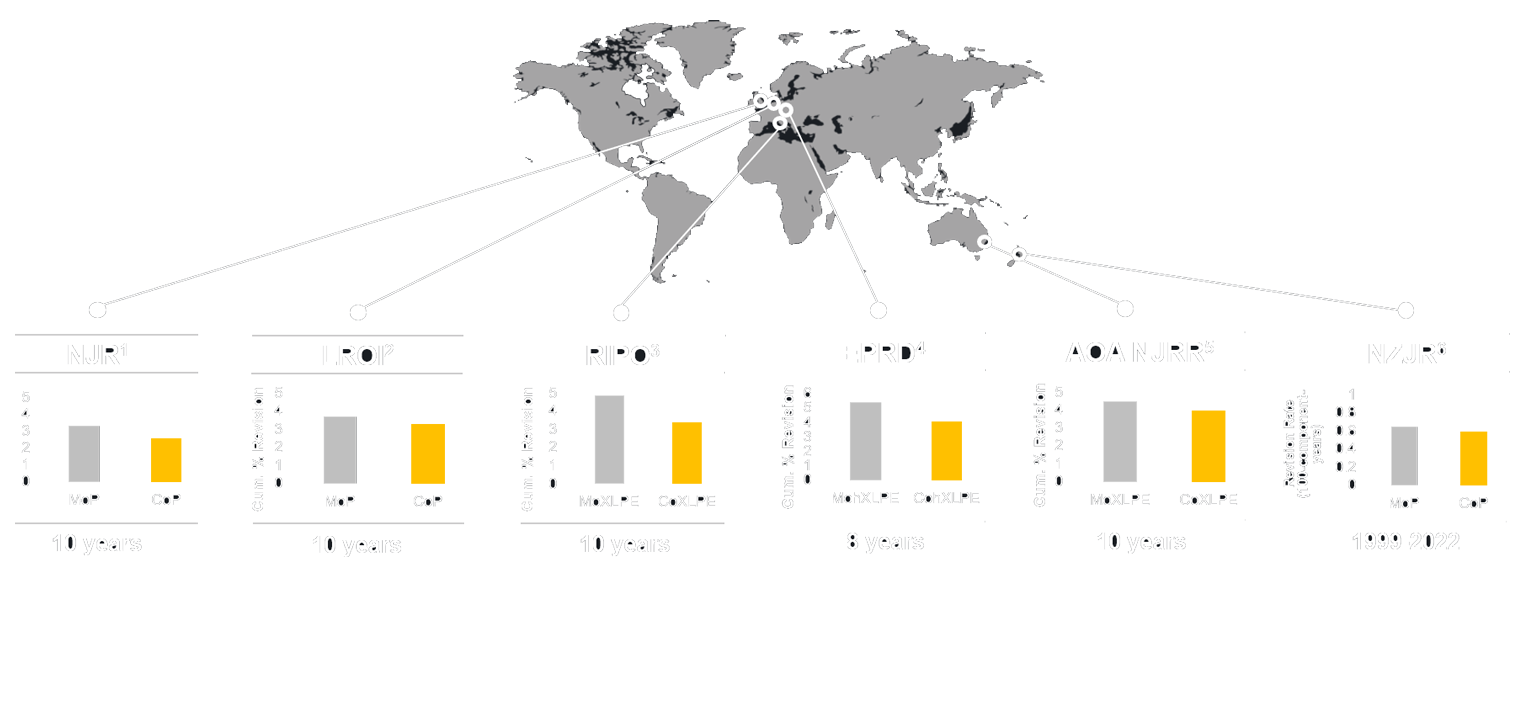
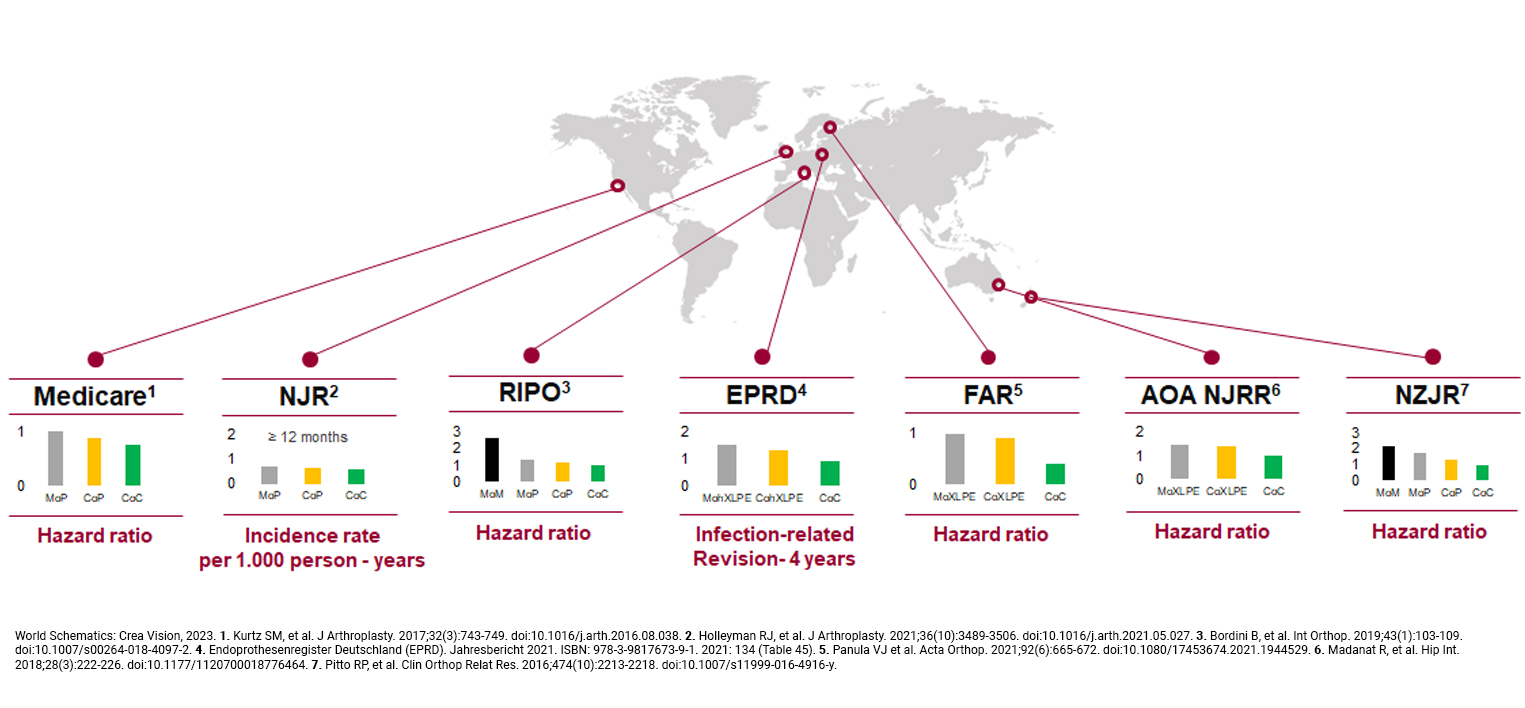
The effectiveness of BIOLOX® ceramics is well-documented in orthopedic registries and scientific studies. These registries, which track the outcomes of various types of joint replacements across countries, consistently show lower revision rates for implants using BIOLOX® ceramics compared to other materials. The data not only highlight the durability and reliability of our products but also underscore our commitment to providing solutions that significantly improve patient outcomes.
FDA Breakthrough Device Designation
Recently the FDA granted Breakthrough Device Designation to three devices made of BIOLOX®delta ceramic:
- CeramTec’s BIOLOX® CERAMIC KNEE
- Matortho’s ReCerf®ceramic-on-ceramic hip resurfacing
- Embody’s H1® ceramic-on-ceramic hip resurfacing
With this decision, the FDA acknowledges the role of BIOLOX®delta ceramic in innovative medical devices. The Breakthrough Device Designation is reserved for products that promise more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases. It highlights our leadership in developing cutting-edge technology and reinforces our role at the forefront of medical advancement. The designation not only prioritizes the review process to bring innovations to market more quickly but also emphasizes the potential of these devices to enhance clinical outcomes.
Continuing to Set Clinical Benchmarks
As we celebrate 50 years of clinical excellence with BIOLOX®, we remain dedicated to advancing the field of joint replacement. Our ongoing research and development efforts aim to further improve the performance and safety of our products. In addition, by continuously refining our technology and processes, we ensure that BIOLOX® remains a leading brand in medical bioceramics, setting benchmarks for clinical performance and innovation.